Introduction
Bipolar disorder types I and II affect about 2% to 4% of the world’s population. Even with treatment, about a third of patients relapse within 1 year, and two-thirds within 2 years.Reference JR and Miklowitz1 According to the Systematic Treatment Enhancement Program for Bipolar Disorder study, relapses of depressive polarity were twice as common as these of the manic polarity.Reference Perlis, Ostacher and Patel2 Moreover, after disease onset, patients experience residual depressive symptoms for about a third of the weeks of their lives.Reference Judd, Akiskal and Schettler3 Thus, depression, and not mania, is considered to be the most problematic phase of bipolar disorder (BD) and it is the most likely cause of long-term disability.Reference Fountoulakis4 Furthermore, bipolar depression appears to be a refractory mental state during which patients may also experience psychotic symptoms, impaired functioning, compromised quality of life, and stigma.Reference Michalak, Livingston and Hole5 Most importantly, it bears a high risk for suicideReference Frye, Gitlin and Altshuler6, Reference Frye, Prieto and Bobo7 and profound and lasting functional impairment.Reference Bonnin, Torrent and Arango8 Interestingly, antidepressants are generally proven ineffective against bipolar depression,Reference Pacchiarotti, Bond and Baldessarini9 and currently available treatments are little efficacious in improving disability and the overall outcomeReference Frye, Prieto and Bobo7, Reference Bauer, Soares and Selek10–12 as residual symptoms may interfere with the ability of the patients to access and benefit not only from healthcare, but also from the general state welfare.13–15 Bipolar depression treatment has proved notoriously difficult for the clinician. All guidelines propose specific or less specific treatment options for acute bipolar depression.Reference Fountoulakis, Grunze and Vieta16–20
Depressive episodes in the course of BD have typically a low rate of response to standard treatment,Reference JR and Miklowitz1 and frequently bipolar depression becomes refractory. Treatment-resistant bipolar depression is common, with approximately one-third of depressed patients failing to achieve symptomatic remission.Reference Rush, Trivedi and Wisniewski21 There are several issues to be addressed before a patient is labeled as refractory, however, including the possibility of misdiagnosis, the presence of somatic or mental comorbidity, nontolerability of medication, or poor compliance with treatment.
Among drugs that are anecdotally used as an add-on for the treatment of bipolar depression, psychostimulants have gained popularity in recent years despite none of them being licensed for this indication. Currently prescribed psychostimulants include methylphenidate (MPH), and the amphetamines, including the prodrug lisdexamphetamine (LDX), metabolized to the active dextroamphetamine in vivo. Modafinil and armodafinil are wakefulness-promoting agents, that is, stimulant-like drugs officially licensed for the treatment of sleepiness due to narcolepsy, shift work sleep disorder, or obstructive sleep apnea. They are most commonly used off-label or unlicensed in the treatment of attention-deficit/hyperactivity disorder (ADHD) and BD.Reference Girard and Joober22, Reference Perugi, Vannucchi and Bedani23
Modafinil and armodafinil appear in the second and fourth steps respectively, of the algorithm suggested by International College of Neuro-Psychopharmacology for the treatment of acute depression in combination with a mood stabilizer,Reference Fountoulakis, Grunze and Vieta16 and as a third-line treatment in bipolar I depression according to the 2018 Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) guidelines,Reference Yatham, Kennedy and Parikh18 as their efficacy and safety in bipolar depression remains poorly understood. A meta-analysis found that modafinil, armodafinil, pramipexole, MPH, and amphetamines may present a useful alternative in the treatment of bipolar depression, with no evidence of a related increase in mood switches,Reference Szmulewicz, Angriman and Samame24 but on the contrary, a second meta-analysis suggested that psychostimulants, namely armodafinil, amphetamine, dextroamphetamine, LDX, MPH, or modafinil, were insufficiently studied for a firm conclusion regarding their use in bipolar depression to be drawn.Reference McIntyre, Lee and Zhou25
Aims
The aim of the current study was to systematically review the literature on the usefulness of add-on LDX, modafinil, and armodafinil in the treatment of resistant acute bipolar depression and subsequently to meta-analyze the available data in terms of efficacy and tolerability/safety.
Experimental Procedures
Literature search
The PRISMA guidelines were followed in the design, organization, and execution of this systematic review and meta-analysis.Reference Moher, Liberati and Tetzlaff26 Literature search was carried out in PubMed/Medline and in the Cochrane Library to February 3, 2020 by using the following key terms: (“bipolar disorder”[MeSH Terms] OR “bipolar disorder” [All Fields] OR “mood disorder”[All Fields] OR “depressive disorder” [All Fields] OR “mania” [All Fields] OR “manic depressive disorder” [All Fields] OR “depression” [All Fields]) AND (“stimulant” [All Fields] OR “stimulants” [All Fields] OR “lisdexamfetamine”[All Fields] OR “modafinil” [All Fields] OR “armodafinil” [All Fields] OR “atomoxetine” [All Fields] OR “dexamphetamine” [All Fields] OR “methylphenidate” [All Fields] OR “dextroamphetamine” [All Fields] OR “amphetamine” [All Fields] OR “psychostimulant*” [All Fields]) AND (“randomized” [All Fields] OR “RCT” [All Fields] OR “trial”[All Fields] OR “randomized-controlled”[All Fields]). A Clinical Trials Search was also performed under the following conditions: Other Terms: efficacy OR safety OR biological effects; Study Type: Interventional; Condition/Disease: Bipolar Disorder OR Mood Disorder OR Depressive Disorder OR Mania OR Manic Depressive Disorder OR Depression; Intervention/Treatment: Stimulant OR Stimulants OR lisdexamfetamine OR atomoxetine OR dexamphetamine OR methylphenidate OR dextroamphetamine OR amphetamine OR psychostimulant OR psychostimulants; Search Results URL: http://tinyurl.com/ya3vkked.
Two authors (E.M.T. and M.M.) examined the extracted articles and decided on study inclusion. The criteria for the inclusion of studies were as follows:
-
• English language.
-
• Double blind randomized trials with placebo arm.
-
• Sample with bipolar depression (no contamination with other diagnoses).
-
• Adult participants (aged 18 years old or older).
-
• Using a psychostimulant or stimulant-like agent in the treatment of acute bipolar depression as therapeutic add-on agent.
-
• Primary outcome is efficacy of treatment.
Collected articles were then thoroughly examined for content to confirm that they were congruent with the inclusion criteria (see flowchart, Figure 1).
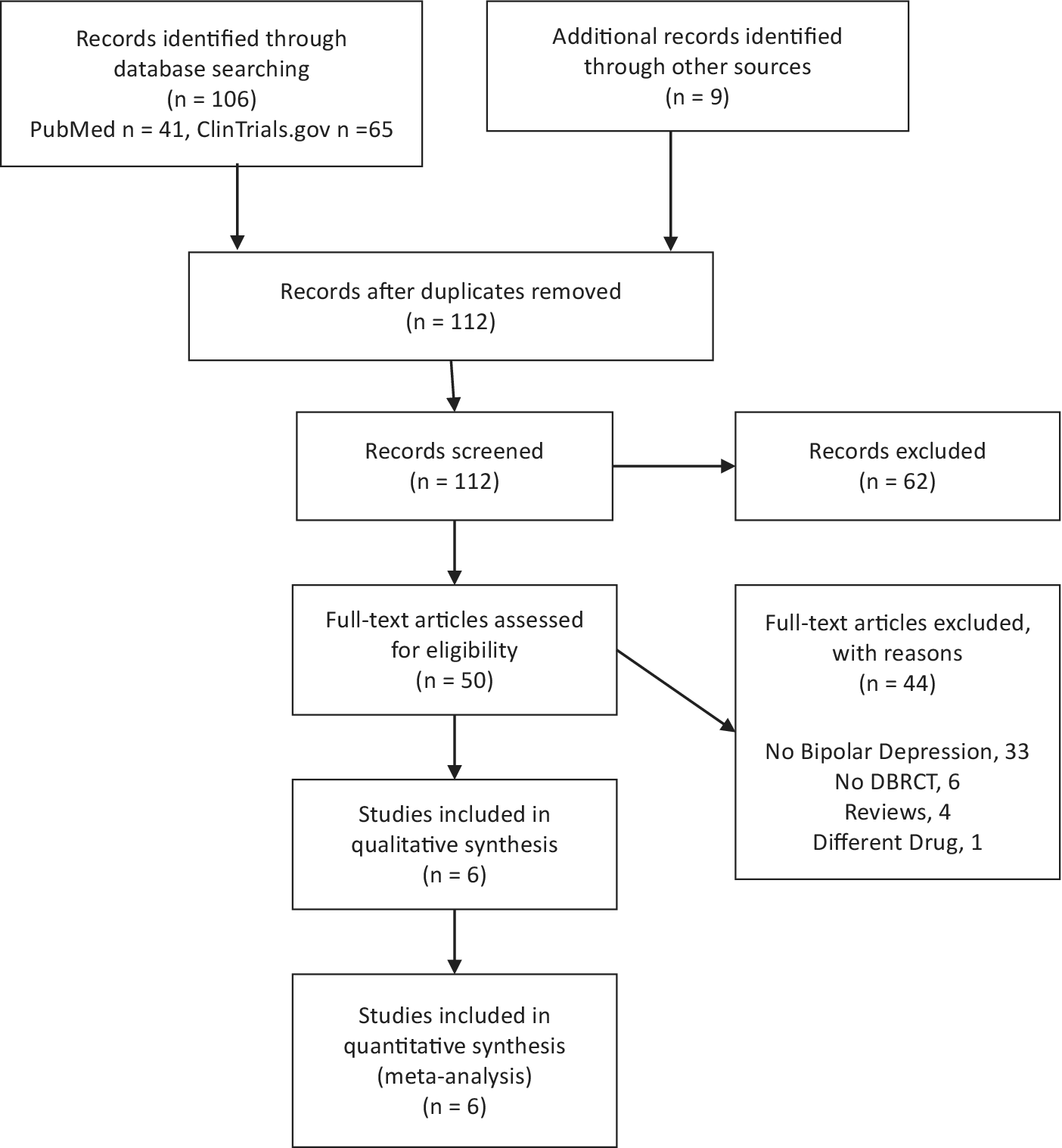
Figure 1. PRISMA flow chart.
The references section of past reviews on the topicReference Fountoulakis, Grunze and Vieta16, 23–25, Reference Goss, Kaser and Costafreda27 and of the retrieved articles was scanned to identify potentially missed studies.
The following data were extracted from the article: location of the study (single or multiple site trial); criteria for diagnosis; criteria for outcome; sample size; mean age in the sample; gender ratio in the sample; duration of the trial; drug used in the trial; information on dropout; and information on anticipated termination of the trial because of adverse events.
Discrepancies in inclusion of articles and in extraction of data were solved by discussion.
Study quality of the Randomized Controlled Trials (RCTs) was evaluated with reference to the Cochrane risk-of-bias tool.Reference Higgins, AD and Jüni28
Data analysis
Included RCTs were based on a pretest–posttest-control design, in which participants were randomly assigned to the treatment or control condition, and each participant was measured both before and after the treatment had been administered.
Relative risk (RR) with 95% confidence interval (CI) was used as effect size, and was calculated on the basis of intent-to-treat.
Two trials included in our meta-analysisReference Calabrese, Frye and Yang11, Reference Ketter, Yang and Frye29 had two treatment arms. In both trials, each treatment arm corresponded to different dosages of the same treatment (armodafinil 150 mg and armodafinil 200 mg). When the trial had two treatment arms with different dosages, the analysis focused on the arm with the largest sample, since the alternative arm included too few cases to be meaningful. The Calabrese et al’sReference Calabrese, Frye and Yang11 study included 33 subjects in the 200 mg armodafinil treatment group, with 24 of them completing the study, and the Ketter et al’sReference Ketter, Yang and Frye29 study included 30 patients in the 200 mg armodafinil treatment group, with 17 participants completing the study. In the Calabrese et al’sReference Calabrese, Frye and Yang11 study, the response rate was 10 out of 33 (30%) against 61 out of 199 (31%) in the placebo, while remission rate was 4/33 (12%) against 34/199 (17%) in the placebo. The Ketter et al’sReference Ketter, Yang and Frye29 study did not report detailed information on response and remission rate in the 200 mg armodafinil arm.
The included studies varied as far as interventions, populations, and outcome measures, thus at preference, the results of the random-effects model were reported for the pairwise meta-analyses as they aim to provide inference about the average effect in the population. Between-studies variance and variance of the effect size parameters across the population were estimated with the tau-squared statistics. The DerSimonian and Laird methodReference DerSimonian and Laird30 was used to fit the random effect model. The heterogeneity variance among studies in the random-effects model was estimated with the empirical Bayes estimator,Reference Morris31 also known as the Paule–Mandel estimator,Reference Paule and Mandel32 and its 95% CI, that was calculated by using the Q-Profile method.Reference Viechtbauer33 This approach is currently considered the best way to estimate the between-study variance.Reference Veroniki, Jackson and Viechtbauer34 The Hartung and KnappReference Hartung and Knapp35 method was used to adjust test statistics and confidence intervals in the estimation of the random-effects model. Approximate prediction interval from the random-effects model for a new study was calculated according to Higgins et alReference Higgins, Thompson and Spiegelhalter36 Prediction interval is helpful to establish whether the results of the meta-analysis will hold or not in future studies.
For the main measure of effectiveness (remission) and the main measure of adverse effects (anticipated termination of the trial because of adverse events), the “number needed to treat for an additional beneficial outcome” (NNT) and the “number needed to treat for an additional harmful outcome” (NNTH) were calculated. The NNT and the NNTH were calculated as the inverse of the absolute risk reduction, which is the difference between the event rate in the control and the event rate in the experimental group, and rounded to the nearest whole number.
To control for adequacy of the models, both the radial plotReference Galbraith37, Reference Galbraith38 and the standardized residuals plotReference Viechtbauer and Cheung39 were considered. For a random-effects model, the radial plot shows the sampling variance of the observed effect size or outcome against the tau-square, that is, the amount of heterogeneity as estimated based on the model. As far as the standardized residuals plot is concerned, if a study fits the model, its standardized residual follows (asymptotically) a standard normal distribution. Therefore, for a study, a large standardized residual (above 2, as a rule of thumb) may suggest that the study does not fit the assumed model (ie, it may be an outlier).
The extent of heterogeneity was assessed using Cochran’s Q and I 2 statistics.Reference Huedo-Medina, Sánchez-Meca and Marín-Martínez40 Results of the heterogeneity assessment are detailed in all reported forest plots (see Figures 2 to 5). Significant Q statistic values (ie, P < .05) were interpreted as suggestive of relevant heterogeneity. For I 2, values between 0% and 40% might not be important; 30% and 60% may represent moderate heterogeneity; 50% and 90%: may represent substantial heterogeneity; and 75% and 100% represent considerable heterogeneity.Reference Higgins, Thompson and Deeks41 Since we retrieved less than 10 independent studies, the funnel plot, and the related statistics,Reference Egger, Davey Smith and Schneider42 was not used.
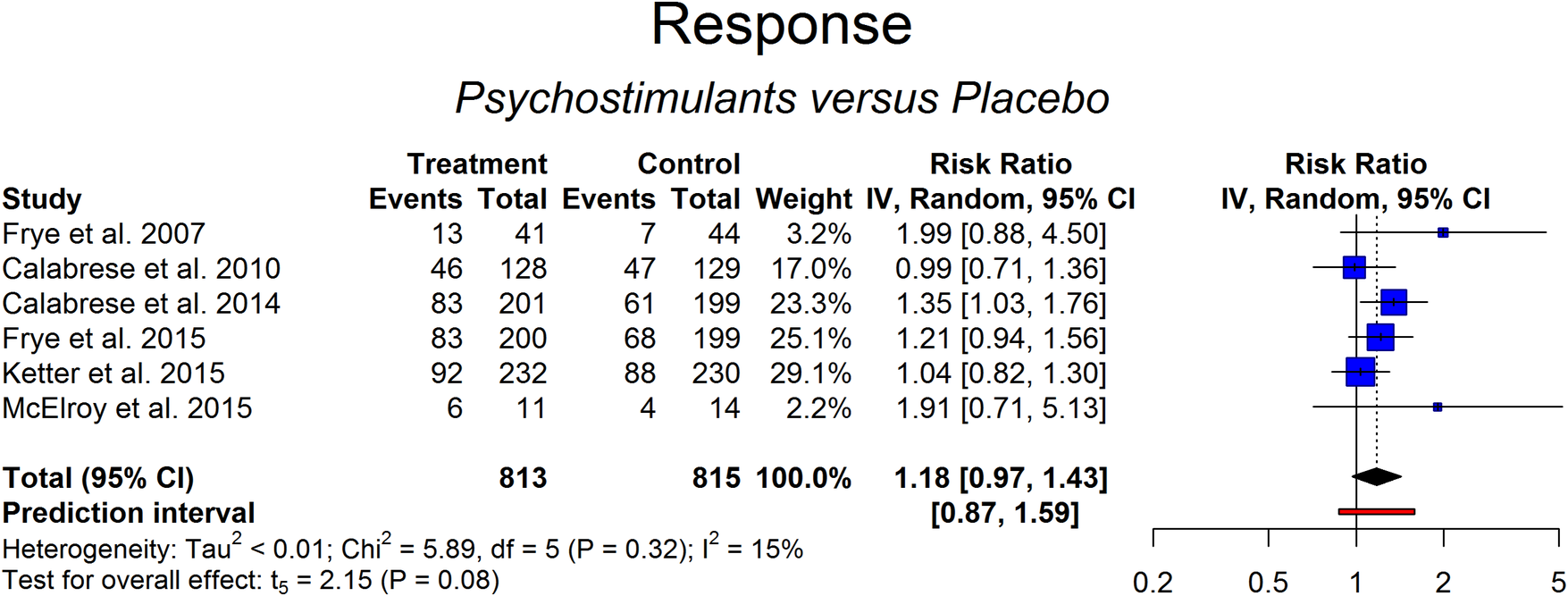
Figure 2. Response of psychostimulants vs placebo.
Risk of bias in the studies was ascertained with the Cochrane risk-of-bias tool for randomized trials.Reference Higgins, AD and Jüni28 We also applied suggestions from the Agency for Healthcare Research and Quality (AHRQ) to evaluate the quality of the studies within a patient safety framework.43
The impact of potential moderators (sample’s composition, duration of the trials, quality rate, etc.) was assessed with meta-regression. All analyses were carried with the “metaphor” packageReference Viechtbauer44 or the “meta” packageReference Schwarzer45 running in R version 3.5.1.Reference Bahji, Ermacora, Stephenson, Hawken and Vazquez46
Results
The PRISMA flowchart is shown in Figure 1. Overall, we found six RCTs of psychostimulants or stimulant-like drugs add-on vs placebo in the treatment of resistant bipolar depression. Our search for trials comparing MPH or amphetamines as add-on treatments in bipolar depression with placebo did not yield any studies which could be included in this meta-analysis.
All, but one study, were multicenter trials. The main characteristics of these studies are summarized in Table 1. In one study, the trial duration was 6 weeks,Reference Frye, Grunze and Suppes47 while in the remaining five trials, the duration was 8 weeks. The study samples included middle-aged patients with the mean age ranging from 42.4 to 44.5 years. The percentage of males ranged from 32% to 84%.
Table 1. Main Characteristics of the Identified RCTs Studying the Use of Psychostimulants as add-on in the Treatment of Bipolar Depression
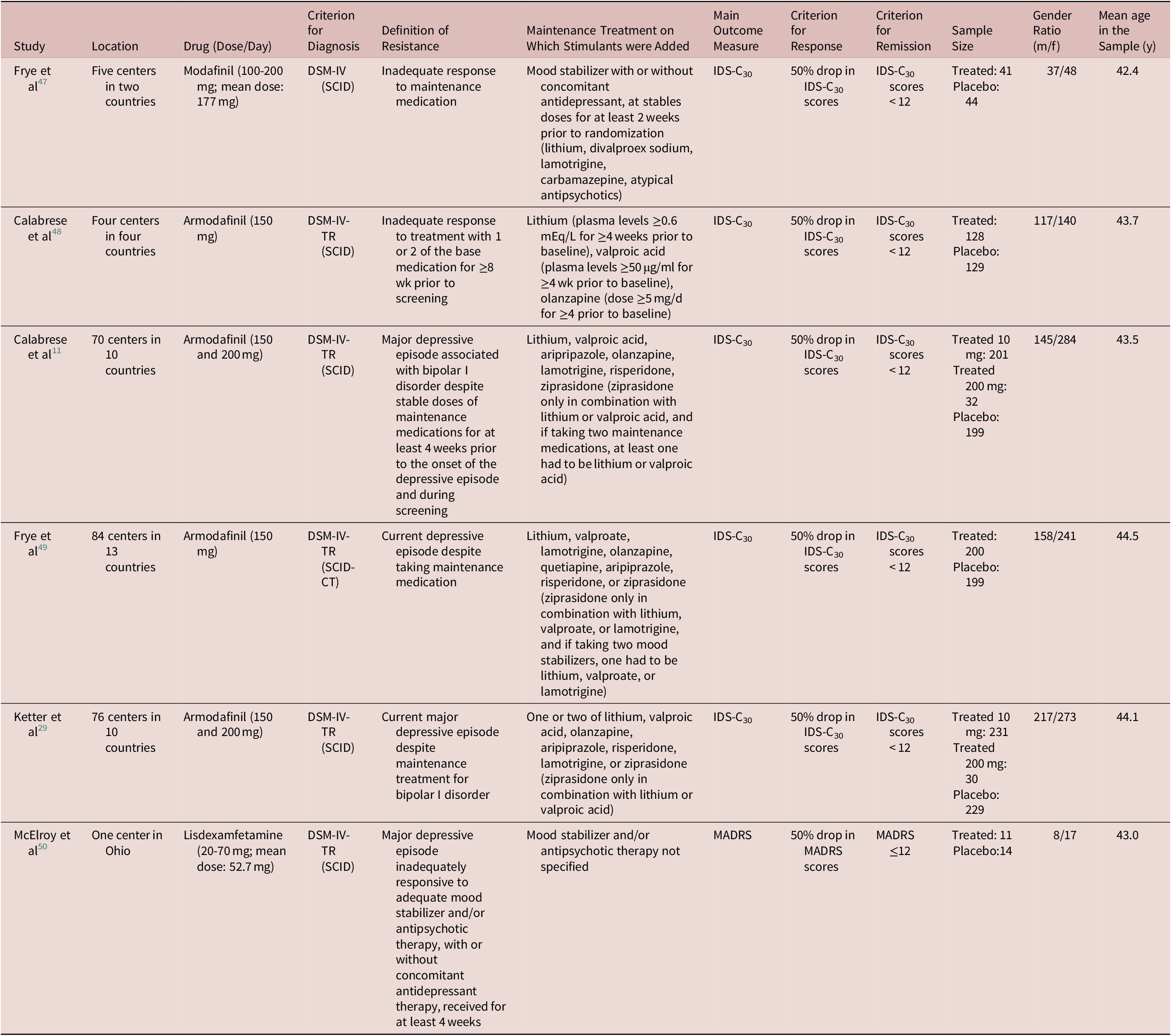
Abbreviations: DSM, Diagnostic and Statistical Manual of Mental Disorders; IDS-C30, 30-item Inventory of Depressive Symptoms (IDS)—Clinician-rated; MADRS, Montgomery and Äsberg Depression Rating Scale; SCID, Structured Clinical Interview for DSM; SCID-CT, Structured Clinical Interview for DSM—Clinical Trials.
The diagnosis of the condition of interest (bipolar depression) was always made according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria in use during the time the study was conducted and on the basis of a standardized interview.
Study quality
Random sequence generation and allocation concealment raised an “unclear risk of bias” in five studies out of six. For all other categories of bias, the studies were judged to have a low risk of bias (Table 2).
Table 2. Assessment of the Risk of Bias in the Included Studies According to the Cochrane Risk-of-Bias Tool for Randomized Trials and the Agency for Healthcare Research and Quality (AHRQ) Guidelines
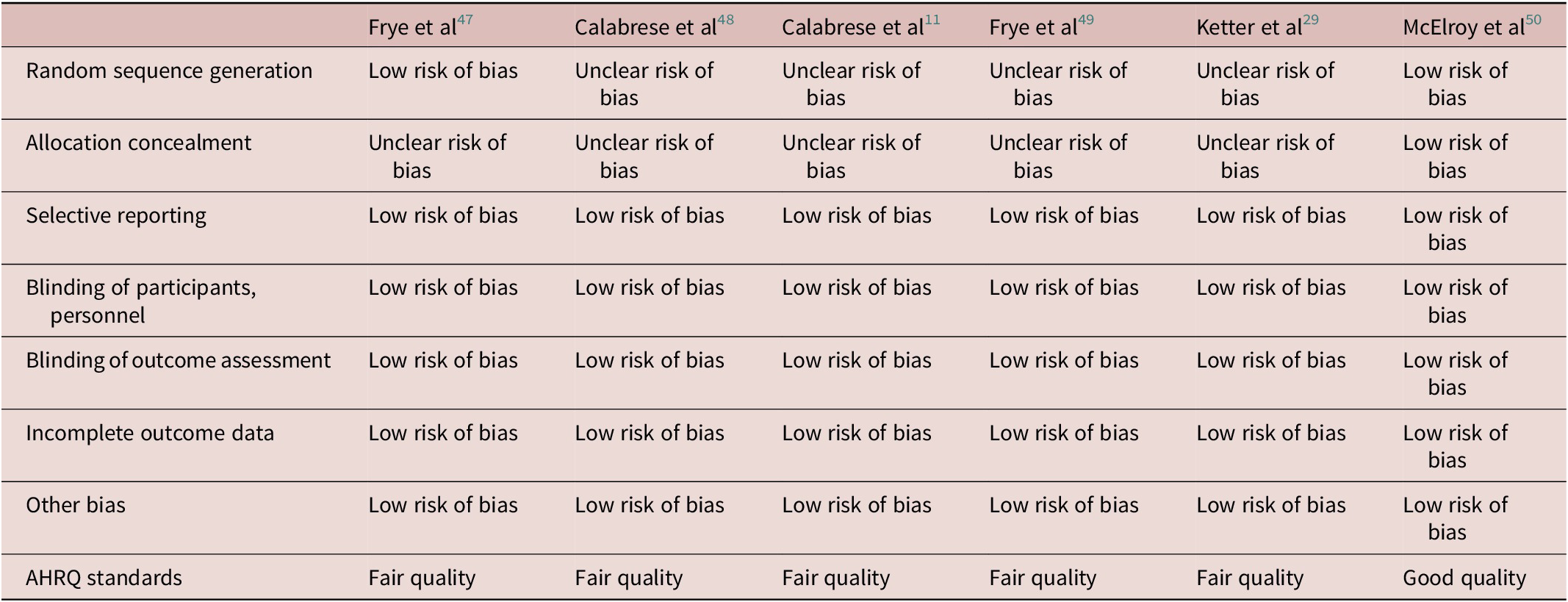
Abbreviation: AHRQ, Agency for Healthcare Research and Quality.
Overall, according to the AHRQ guidelines, one studyReference McElroy, Martens and Mori50 was judged to be of good quality, the other five studies were judged of fair quality. The study with the highest quality according to the AHRQ guidelines had the highest response and remission rates but the lowest sample size.
Efficacy (response and remission rate)
Six studies were included in the evaluation of response and remission rate in 813 patients treated with psychostimulants or stimulant-like drugs and 815 controls under placebo. The criterion used in the studies to assess response to treatment was a 50% drop in the scores of the main outcome, whatever the reached score. According to this criterion, the response rate ranged from 16% to 38% in the placebo arm and from 30% to 55% in the active treatment arm. The results of the random-effects model (RR = 1.18 [0.97-1.43], P = .08; prediction interval: 95%CI of RR: 0.87-1.59) suggested that response rate was not higher in patients treated with psychostimulants or stimulant-like drugs in comparison to placebo (Figure 2). Heterogeneity was low but with large confidence intervals (0%-78%; Cochran’s Q: P > .05). No outlier was detected (Figures A1 and A2 in Supplementary Material).
In order to define remission, a more stringent criterion was used, namely a drop in the scores of the main outcome measure below a nonclinical threshold. According to this criterion, the remission rate ranged from 11% to 29% in the placebo arm and from 12% to 55% in the active treatment arm. The results of the random-effects model (RR = 1.37 [1.06-1.77], P = 0.02; NNT = 16) indicated that psychostimulants or stimulant-like drugs were more likely than placebo to induce remission (Figure 3). The radial and the standardized residuals plot did not reveal outliers (Figures A3 and A4 in Supplementary Material). Heterogeneity was low but with large CIs (0%-73%; Cochran’s Q: P > .05). Prediction interval from the random-effects model (95% CI of RR: 1.04-1.81) indicated that the result would hold in future studies.
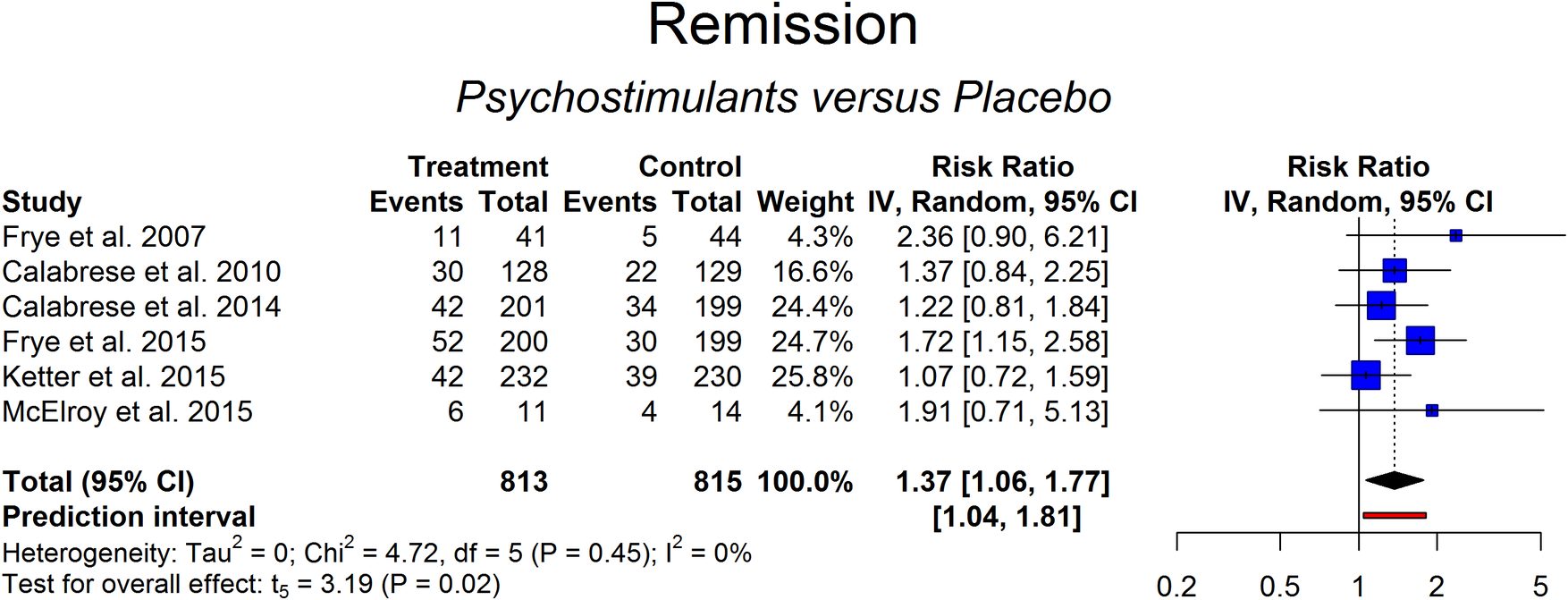
Figure 3. Remission achieved with psychostimulants vs placebo.
Tolerability (dropout rates)
Data on dropout was available for the six studies. Percentage of dropout ranged from 16% to 34% in the placebo arm and from 9% to 30% in the active treatment arm. Results of the random-effects model (RR = 1.04 [0.91-1.18], P = .50) indicated that treatment with psychostimulants or stimulant-like drugs was not associated with a higher risk of dropout compared to placebo (Figure 4). Heterogeneity across studies was low (95 CI: 0%-17%; Cochran’s Q: P > .05). No outliers were detected (Figures A5 and A6 in Supplementary Material).
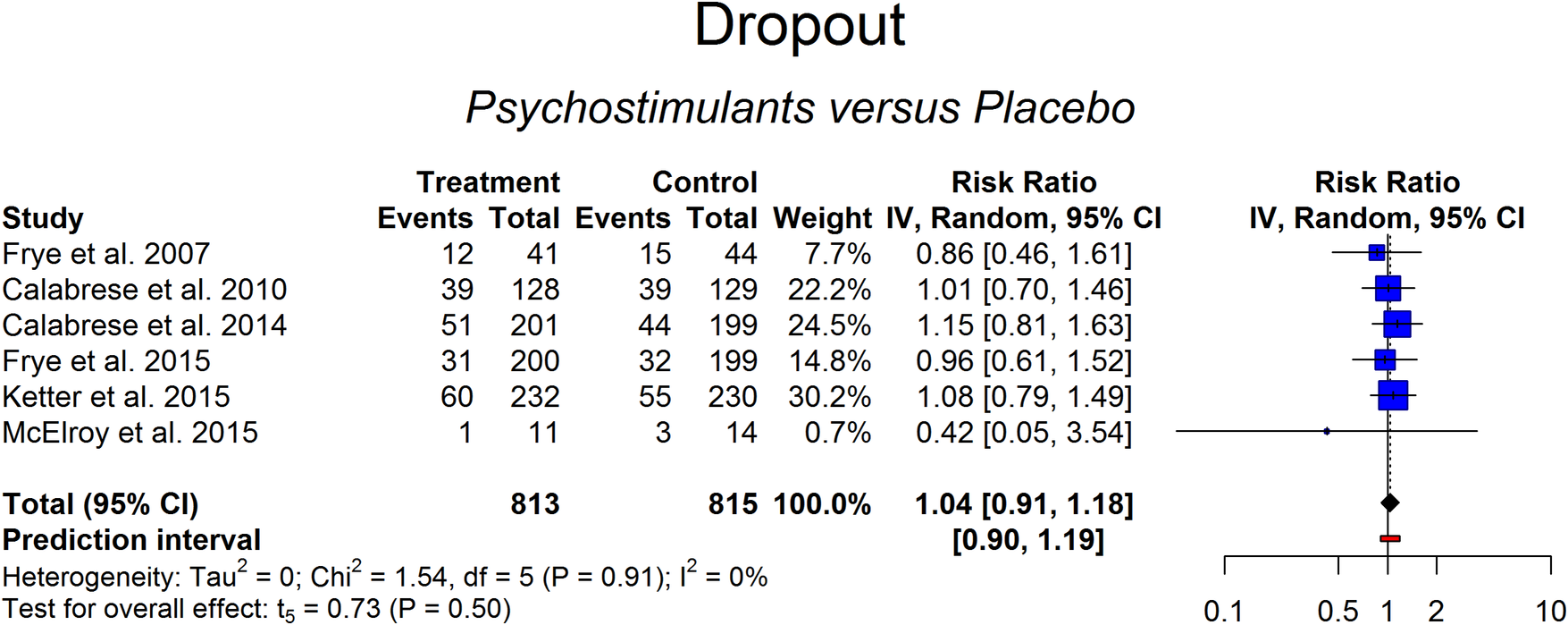
Figure 4. Dropouts in the psychostimulant vs placebo groups.
Safety (side effects and anticipated termination of the trial because of adverse events)
Headache, insomnia, and hypomania were the most frequently reported side-effects in the included studies, followed by nausea and anxiety, which were reported in five studies (Table 3). Most side-effects or adverse events were equally likely in the experimental and the placebo arm group, with some exceptions (Table 4). There were two occurrences of acute urticarial or pruritic rash in the experimental group against none in the placebo group (NNTH = 7). Nausea was more likely in the experimental group, being reported by 35 participants out of 685 in 5 studies that recorded it, compared to 23 out of 686 in the placebo group (NNTH = 59). Conversely, dyspepsia was reported by 4 participants out of 58 in the placebo arm in 2 studies that recorded it as compared to none in the experimental group. People in the experimental group were more likely to report a significant decrease (≥7%) in body weight (NNTH = 30) and less likely to experience a significant increase (≥7%) in body weight than those in the placebo arm (details in Tables 3 and 4).
Table 3. Side or Adverse Events Across the Identified RCTs Studying the use of Psychostimulants as Add-On in the Treatment of Bipolar
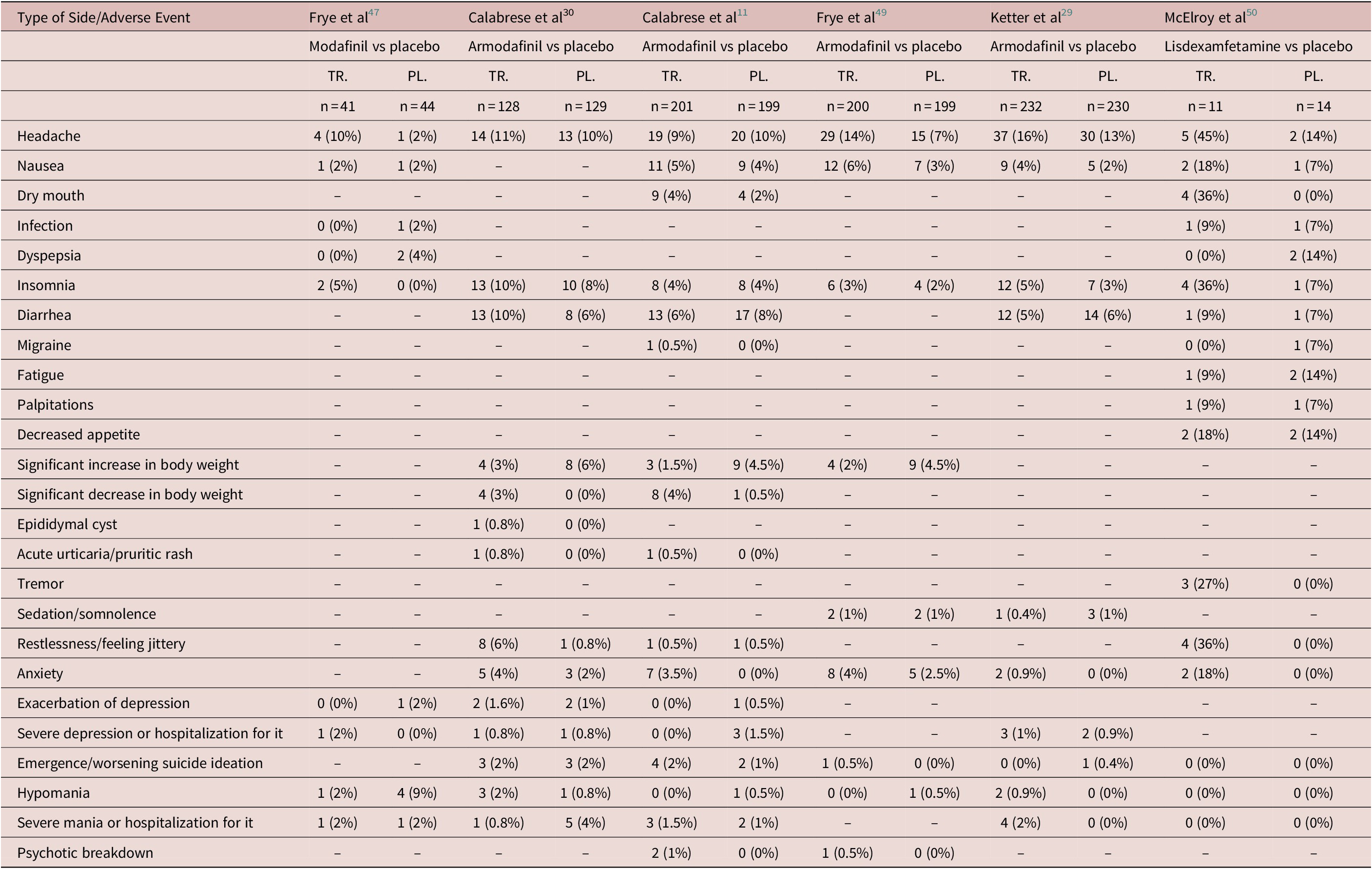
Abbreviations: PL., placebo; TR., treatment (active drug); –, unreported.
Table 4. Results of Random-Effects Models of Adverse Events Across the Identified RCTs Studying the Use of Psychostimulants as Add-On in the Treatment of Bipolar Depression
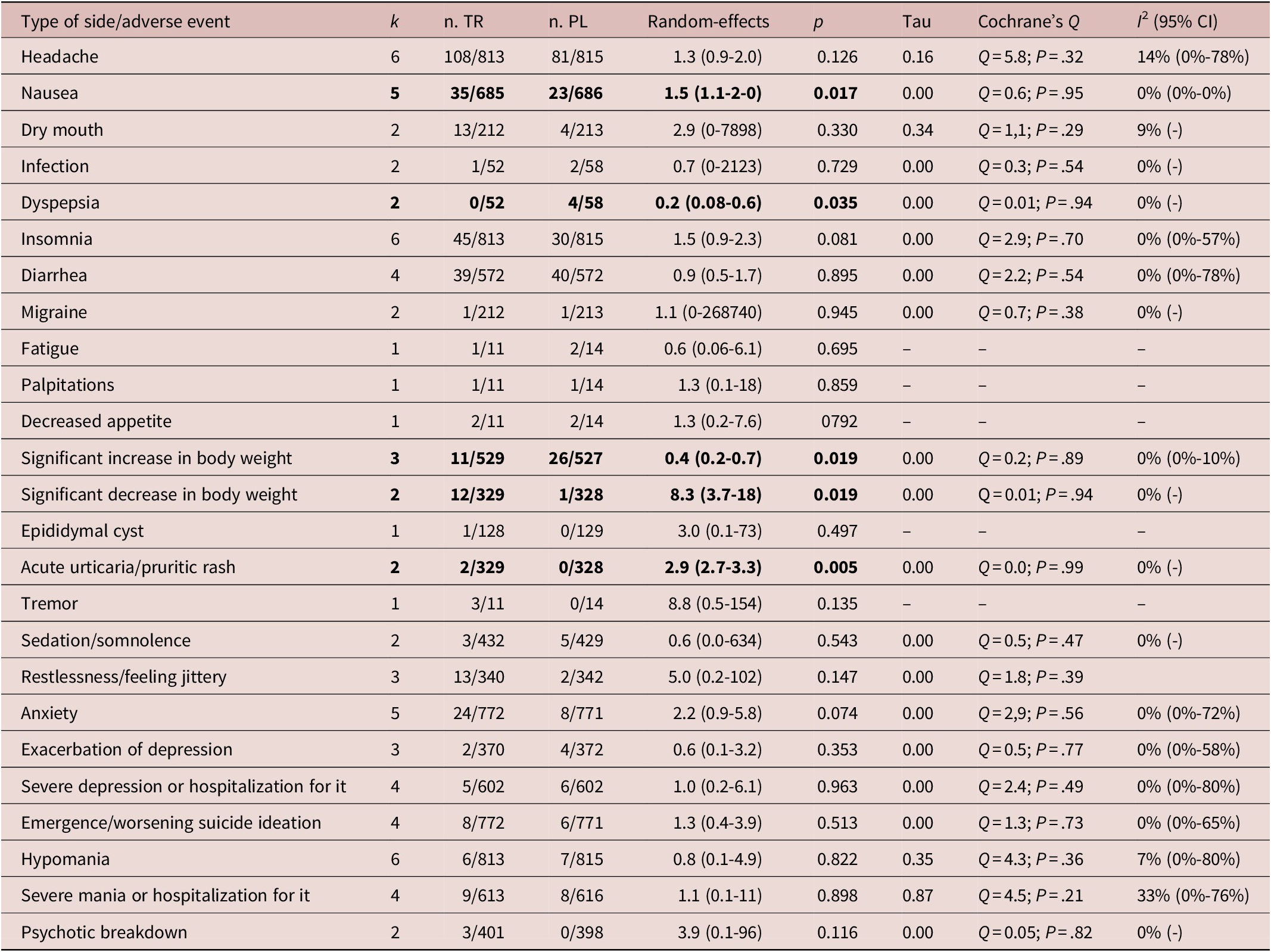
Statistically significant results are in bold. Sample size varies according to the numerosity of the included studies.
Abbreviations: CI, confidence interval; n, number of events/sample size; PL., placebo; TR., treatment (active drug).
Emergence or worsening of suicide ideation or manic switch was not more frequent in the experimental than in the placebo group (Table 4). Overall, LDX, modafinil, and armodafinil were reasonably free of side-effects at the prescribed doses.
Details on severe adverse effects were available for five studies including 772 patients treated with these agents and 771 under placebo. Percentage of anticipated termination of the trial because of adverse events ranged from 4% to 9% in the placebo arm and from 0% to 13% in the active treatment arm. Results of the random-effects (RR = 1.30 [0.81-2.10], P = .20) model indicated that there was not a higher risk of adverse events leading to an anticipated termination of trial in patients treated with the stimulants studied in comparison to placebo (Figure 5). The number needed to treat for an additional harmful outcome was 62, which is fairly safe. Heterogeneity was low but with large confidence interval (0%-71%; Cochran’s Q: P > .05). No outliers were detected (Figures A7 and A8 in Supplementary Material).
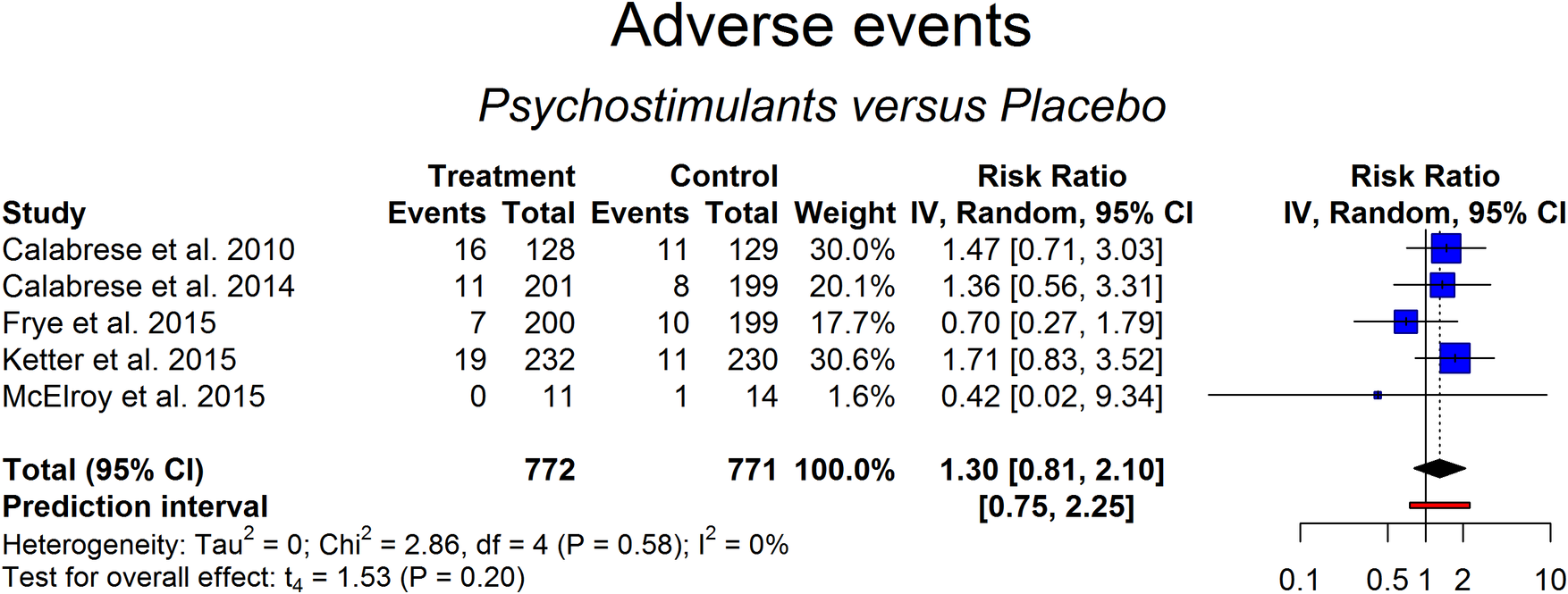
Figure 5. Adverse events analysis between subjects treated with add-on psychostimulants vs the placebo-treated group.
Impact of moderators
The sample composition by sex or age did not influence outcome in any of the performed meta-analyses (P > .05 in all meta-regression). No effect was found for additional potential moderators, such as total sample size, duration of treatment, or the drug that was used in the trial.
Discussion
The current meta-analysis provides some evidence in support of the usefulness of LDX and the stimulant-like agents modafinil and armodafinil as an add-on to treatment as usual in bipolar depression. Treatment as usual refers to treatment used clinically that presents variable degrees of effectiveness.Reference Bahji, Ermacora, Stephenson, Hawken and Vazquez46 The following outcomes were considered: response and remission, as defined by the authors in each study included in the meta-analysis; tolerability, as measured by dropout (anticipated termination of the trial for any cause); safety, as measured by side-effects and anticipated termination of trial because of adverse events.
The stimulants studied here were more likely to induce remission from an episode of resistant bipolar depression compared to placebo but did not differ from placebo in terms of response. Overall, they imported a greater risk of nausea (5.1% vs 3.3%) and a significant decrease in body weight (3.6% vs 0.3%). Side-effect frequency did not differ significantly between experimental and placebo arms of the trials. Interestingly, this report failed to show higher rates of emergence of hypomania or mania due to LDX, modafinil or armodafinil add-on to treatment-as-usual for resistant bipolar depression. Adding-on LDX, modafinil, and armodafinil did not result in more dropouts compared to placebo, neither was there a higher risk of adverse events or of emergence/worsening of suicide ideation, suggesting that these drugs offer a reasonably well-tolerated and safe treatment.
All three compounds enhance dopaminergic prefrontal transmission, through different mechanisms of action, however.
LDX, a prodrug of d-amphetamine, blocks the reuptake of norepinephrine (NE) and dopamine (DA) into the presynaptic neuron by reversing the direction of the dopamine and norepinephrine transporter pump (Dopamine Transporter [DAT] and Norepinephrine Transporter [NAT]) resulting in the active diffusion of neurotransmitters in the synaptic cleft. It also inhibits the vesicular monoamine transporter (VMAT-2) inside the cell favoring extracellular diffusion of DA.Reference Sonders, Zhu, Zahniser, Kavanaugh and Amara51 More specifically, by targeting the trace amine-associated receptor 1 (TAAR1), which interacts with both presynaptic and post-synaptic D2 receptors to synergistically control the downstream signaling pathways, LDX increases synaptic concentrations of these catecholamine neurotransmitters in the prefrontal cortex, and in the striatum.Reference Liu and Li52 Amphetamines also enhance dopaminergic transmission not only in the reticular activating system and the prefrontal cortex, but also in the nucleus accumbens, which is responsible for their addictive potential and the worsening or emergence of tics. Abuse liability varies, however, with the delivery system used in these formulations.Reference Mansbach and Moore53 Conversion of LDX to its active component requires enzymatic hydrolysis that results in a slow rise in serum d-amphetamine level, which possibly provides a reduced potential for abuse.Reference Jasinski and Krishnan54 In a human abuse-liability study involving individuals with a history of drug abuse, lisdexamfetamine produced subjective responses that were significantly less than an equivalent oral dose of d-amphetamine immediate release.Reference Elia, Easley and Kirkpatrick55 Modafinil and armodafinil seem to act mostly at the suprachiasmatic nucleus in the hypothalamus and at the amygdala, both regions primarily involved in wakefulness. So, their interaction with the nucleus accumbens is believed to be less relevant than that of the amphetamines, hence allowing them to bear reduced addictive potential.Reference Vosburg, Hart, Haney, Rubin and Foltin56
It has also been debated that the stimulation of nucleus accumbens is involved in the induction of (hypo)manic switches, cycle acceleration, and psychosis, often related with the use of amphetamines.Reference Young and Dulcis57 Modafinil and its R-enantiomer armodafinil are stimulant-like agents promoting DA- and NE-related transmission. Differences with psychostimulants include their low affinity for the DAT.Reference Madras, Xie and Lin58, Reference Schmitt and Reith59 In addition, modafinil increases noradrenergic transmission by directly acting as a partial agonist on alpha-1B adrenergic receptors, decreases gamma-amino-butyric Acid (GABA) release and increases glutamate release in various brain regions. It also activates orexigenic hypothalamic neurons and the histaminegic (H1) tuberomammillary nucleus.Reference Chen, Qu and Qiu60, Reference Minzenberg, Watrous, Yoon, Ursu and Carter61 Armodafinil is not a direct- or indirect-acting DA receptor agonist. However, in vitro, armodafinil is an antagonist to the DAT and inhibits DA reuptake, thus enhancing DA neurotransmission.Reference Vosburg, Hart, Haney, Rubin and Foltin56
The discrepancy between response and remission in patients with resistant bipolar depression treated with the three drugs studied may depend on them being able to affect only a subgroup of patients, possibly more sensitive to the action of the drug. As a consequence, when evaluated as a generic 50% drop in the outcome scores, the average effect was found to be similar in patients under the active treatment and patients receiving placebo. However, when a prespecified threshold is used, such as an IDS-C30 score < 12, as in most studies, more patients under the active treatment were found to achieve this threshold than patients receiving placebo.
Serotonin reuptake inhibition does not seem to play a major role for acute bipolar depression in contrast to unipolar depression, as antidepressants seem to be largely ineffective in the former.Reference Pacchiarotti, Bond and Baldessarini9 Furthermore, it has been suggested that NE reuptake and 5-HT1A agonism along with hypoactive NE and serotonin neurotransmission may play a central role in bipolar depression, and that NE rather than serotonin activity seems to be more important in bipolar depression.Reference Fountoulakis, JR and Akiskal62 Furthermore, evidence suggests that increased striatal DAT levels lead to reduced dopaminergic function and depression, postulating that a failure of DA receptor and transporter homoeostasis possibly underlies the pathophysiology of bipolar depression.Reference Ashok, Marques and Jauhar63 Currently, Food and Drug Admnistration (FDA)-approved treatments for bipolar depression are the olanzapine–fluoxetine combination, quetiapine, lurasidone, and cariprazine. They are all D2 and D3 antagonists, but cariprazine is a strong D3 partial agonist.Reference Stahl64 Cariprazine’s binding affinity to the D3 receptors is higher than DA’s affinity for this receptor, meaning that, at physiological doses, DA cannot reverse D3 binding.Reference Stahl, Laredo and Morrissette65 With regard to the serotonin system, all four antipsychotics are antagonists at the 5HT7 receptors in the hypothalamus, and fluoxetine results in 5HT7 downregulation at the SCNReference Mullins, Gianutsos and Eison66; lurasidone, quetiapine, and cariprazine are partial agonists at the 5-HT1A receptors, and antagonists at the 5HT2A receptors, whereas olanzapine is an antagonist at 5-HT1A and an inverse agonist at 5HT2A receptors.Reference Bymaster, Calligaro and Falcone67 Olanzapine, quetiapine and fluoxetine block the norepinephrine transporter (NET) and all antipsychotics in this group also enhance cortical cognitive function by exerting inhibitory effects at alpha-1 receptors.Reference Arnsten, Mathew, Ubriani, Taylor and Li68 All, except lurasidone inhibit muscarinic and H1 histamine receptors.Reference Fountoulakis, Gazouli and Kelsoe69 According to the model for antidepressant pathways in bipolar depression suggested by Fountoulakis et al,Reference Fountoulakis, JR and Akiskal62 serotonin reuptake is neither sufficient nor necessary as a condition for the antidepressant efficacy in bipolar depression. Moreover, no class effect seems to exist in the treatment of bipolar depression and this primarily concerns antidepressants.Reference Fountoulakis, Gonda and Vieta70 NE activity, however, seems to be absolutely necessary in order to sustain the antidepressant effect.Reference Fountoulakis, JR and Akiskal62 Furthermore, it has previously been suggested that a regionally selective balance between the DA and serotonin systems may account for the mood-stabilizing properties of atypical antipsychotics such as quetiapine and olanzapine.Reference Brugue and Vieta71 It is, therefore, possible that the psychostimulant and stimulant-like drugs studied here exert their antidepressant effect in bipolar depression by mediating increases in NE and DA in the extracellular space, in a similar way to the evidence-based treatments for bipolar depression.Reference Grunze, Vieta and Goodwin17
Implications for clinical practice
Our results replicate these of an earlier meta-analysis,Reference Szmulewicz, Angriman and Samame24 which examined the effects of dopaminergic agents used in ADHD and the dopamine agonist pramipexole used in Parkinson’s disease, as add-on treatments in acute bipolar depression. Moreover, we also replicated the results of a more recent meta-analysis showing that, compared to placebo, augmentation with modafinil/armodafinil (MoArm) as adjunctive treatment for bipolar depression was associated with significantly greater rates of treatment response, with the all-cause discontinuation not differing from placebo and no evidence of mood switch or suicide attempts on MoArm treatment.Reference Nunez, Singh and Romo-Nava72
Based on the above, in addition to using psychostimulants in comorbid ADHD and BD, it seems that using psychostimulants (and stimulant-like drugs) as an add-on to standard treatment in resistant bipolar depression may well have a place in clinical practice, not only in in-patients but in out-patients as well. Improvement in the symptoms of resistant bipolar depression translates into improvement in patient quality of life, decreased carer and family burden, lower cost for the health service and fewer lost days at work.Reference Manning73
The use of S-ketamine in resistant depression is being discussed extensively in recent years, and FDA approval for its use in treatment-resistant depression was granted in February 2019. S-ketamine, however, is complicated in its mode of administration, bears an addiction potential and is marketed as an expensive treatment that many health services around the globe are unable to offer to their patients. Furthermore, it appears to be less effective in bipolar compared to unipolar resistant depression.Reference Kraus, Rabl and Vanicek74, Reference Canuso, Singh and Fedgchin75
Nevertheless, our results should be further replicated by multicenter longitudinal randomized clinical trials, with a much larger number of participants diagnosed by strict consensus criteria for resistant bipolar depression, and with a more extended follow-up period to reflect the chronicity of bipolar depressive episodes. Moreover, the use of assessment tools and questionnaires to assess and follow-up symptomatology should be revised to include newer and more specific instruments, such as for example, the Koukopoulos Mixed Agitation Depression Rating Scale.Reference Canuso, Singh and Fedgchin75 In addition, head-to-head trials comparing psychostimulant and stimulant-like medications with well-established approved effective treatments in resistant bipolar depression would also help to clarify the role of add-on stimulant medication further.
Implications for research
Based on the results of this meta-analysis, further research should be aimed toward identifying the specific subsample of treatment-resistant bipolar depression patients for which add-on treatment with psychostimulants and stimulant-like medications is efficacious, if indeed such a subsample exists. Moreover, a better understanding of the mode of action of psychostimulants and stimulant-like medications in acute bipolar depression will inform further pharmacological hypotheses on the neurochemistry and the aetiology of depression in BD as well as on the possible causes of resistant depression in BD.
Conclusions
The current study provides some limited data concerning the usefulness of add-on stimulants in resistant bipolar depression. Remission but not response rates improve in comparison to placebo, suggesting a selective effect on a subgroup of patients. In view of the potential benefits of add-on psychostimulant and stimulant-like medication to treatment as usual in patients with acute bipolar depression, this study suggests a possible revision of treatment guidelines to include modafinil, armodafinil and LDX earlier in the therapeutic algorithm for resistant acute bipolar depression. To date, there is no study investigating the effectiveness of add-on psychostimulant and stimulant-like medication in comparison with an active compound. Therefore, further research is necessary in order to elucidate the usefulness of these and other similar compounds and their clinical applicability.
Supplementary Materials
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S109285292000156X.
Disclosures
Evangelia Maria Tsapakis, Antonio Preti, and Michael Mintzas have nothing to disclose. Konstantinos Fountoulakis has received grants and served as consultant, advisor or CME speaker for the following entities: AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Ferrer, Gedeon Richter, Janssen, Lundbeck, Otsuka, Pfizer, the Pfizer Foundation, Sanofi-Aventis, Servier, Shire, GAP, and Rafarm.











