Introduction
Clay minerals are currently commonly employed in catalysis because they are inexpensive, abundant in nature, and environmentally friendly. However, their usefulness is restricted due to a lack of porosity and low thermal stability. As a result, adjustments are made to improve these properties, including acid treatment and the pillaring process (Bineesh et al., Reference Bineesh, Kim and Park2010b). Acid treatment is a useful approach in enhancing the catalytic activity of clay minerals (Komadel and Madejová, Reference Komadel and Madejová2006). Acid-modified clay minerals are being used widely in the following ways: disposal of radioactive waste (Wang et al., Reference Wang, Zhu, Ge, Zhu, He and Chen2010); removal of heavy metals from industrial wastewaters (Oubagaranadin et al., Reference Oubagaranadin, Murthy and Mallapur2010); and as catalysts or catalyst supports (Chitnis and Sharma, Reference Chitnis and Sharma1997; Rhodes et al., Reference Rhodes, Franks, Parkes and Brown1991). On the other hand, the pillaring process, during which rigid metal-oxides are inserted or intercalated between the clay mineral layers, increases the specific surface area of the solid which improves the performance of low-surface-area catalysts by adding to the pillared interlayer clays (PILC) (Torres et al., Reference Torres, los Santos, Portugau, Yeste and Castiglioni2021). PILC materials, thus, have attracted much interest and are an active research subject (Centi and Perathoner, Reference Centi and Perathoner2008; Najafi et al., Reference Najafi, Farajfaed, Zolgharnian, Mosavi Mirak, Asasian-Kolur and Sharifian2021; Pinnavaia, Reference Pinnavaia1983). Several studies have been devoted to PILCs, reviewing various aspects of their preparation, characterization, and application. Because of their porosity, thermal stability, acidity, and reactivity, PILCs are used widely as catalysts or supports for catalytic materials and applied in several chemical processes (Centi and Perathoner, Reference Centi and Perathoner2008; Huang et al., Reference Huang, Zuo and Zhou2010; Pinnavaia, Reference Pinnavaia1983). Recently, many studies have shown that vanadia supported on Ti-pillared clay (V/Ti-PILC) is more active than Ti-PILC in various reactions such as selective catalytic oxidation of H2S (Bineesh et al., Reference Bineesh, Kim, Jermy and Park2009); ammoxidation of m-xylene (Bahranowski et al., Reference Bahranowski, Dula, Komorek, Romotowski, Serwicka, Poncelet, Martens, Delmon, Jacobs and Grange1995), selective catalytic reduction of NO by NH3 (Bahranowski et al., Reference Bahranowski, Janas, Machej, Serwicka and Vartikian1997; Chae et al., Reference Chae, Nam, Ham and Hong2004; Long and Yang, Reference Long and Yang2000a), oxidative dehydrogenation of propane (Bahranowski et al., Reference Bahranowski, Grabowski, Grzybowska, Kielski, Serwicka, Wcisło and Wodnicka2000), and epoxidation of allylic alcohol over vanadium-supported Ti-pillared clay (Arfaoui et al., Reference Arfaoui, Boudali and Ghorbel2010). Epoxidation reactions are one of the key reactions in the chemical industry promoting the conversion of olefins into oxygenated molecules, referred to as epoxides, by an oxygen transfer reaction. Epoxides are important and versatile commercial intermediates used as key raw materials for a wide variety of products due to the many reactions they can undergo (Sreethawong et al., Reference Sreethawong, Yamada, Kobayashi and Yoshikawa2005). The epoxidation of cyclohexene to yield cyclohexene oxide as the main product is one of the most difficult to achieve of these types of reactions, for two main reasons, i.e. allylic oxidation and epoxy ring opening occur readily and interfere with epoxidation (El-Korso et al., Reference El-Korso, Khaldi, Bedrane, Choukchou-Braham, Thibault-Starzyk and Bachir2014). Cyclohexene oxide is an important organic intermediate used in the production of pharmaceuticals, plant protection agents, pesticides, and stabilizers for chlorinated hydrocarbons (Gao et al., Reference Gao, Chen, Han, Feng, Li, Zhou and Xi2004; Parida and Mallick, Reference Parida and Mallick2009). Much effort is invested in the development of novel active and selective cyclohexene epoxidation catalysts that avoid side reactions and the subsequent formation of large amounts of unwanted by-products (El-Korso et al., Reference El-Korso, Bedrane, Choukchou-Braham and Bachir2016). In an attempt to enhance the catalytic performance of bentonite, the present study aimed to address the inefficiency of bentonite as a catalyst due to its low acidity and low catalytic activity by first exposing it to acid treatment then incorporating transition metals, specifically titanium and vanadium, in order to impart to it Brønsted and/or Lewis acidity and oxidation-reduction properties. A further objective was to understand better the correlation between the physicochemical properties of this catalyst and its performance in the epoxidation of cyclohexene.
Materials and methods
Raw materials and preparation of catalysts
In the present study, a Maghnia (Algeria) bentonite was utilized as the primary clay material. Before its application, the raw bentonite underwent a purification process (120 g of bentonite was dispersed in 1.5 L of distilled water in a 5 L beaker with stirring for 15 min). Then, a buffer solution (0.3 M sodium citrate, Hebei Fengqiang Trading, China, 99%), 1 M sodium bicarbonate, and 2 M sodium chloride (Aldrich, 99%) at pH = 7.3 was added to the bentonite. After that, the mixture was heated with stirring at a temperature of 75°C for 20 min. 15 g of sodium thiosulfate, Na2S2O3(Prolabo, 99%), was then added slowly. After 15 min of stirring, a further 15 g of Na2S2O3 (Prolabo, 99%) was added. The cooled mixture was centrifuged using a Rotofix 32A Hettich centrifuge (Andreas Hettich, Germany), at (6000 rpm, 4226×g) for 15 min. The bentonite was washed twice with 0.05 M HCl (1.5 L) (Biochem Chemopharma, Cosne-Cours-sur-Loire, France, 37%) for 3–4 h. After centrifugation (6000 rpm, 4226×g), the bentonite was redispersed in 2.5 L of hydrogen peroxide (H2O2: 10 volumes) (Sigma-Aldrich, 30%) overnight, then heated at 70°C for 30 min to remove the organic matter. The cation exchange capacity (CEC) was measured in a previous study (Belaidi et al., Reference Belaidi, Bedrane, Choukchou-Braham and Bachir2015) by exchanging cobalthexamine cations (Aldrich, 99%), then analyzed by UV-vis using the procedure of Ciesielski and Sterckeman (Reference Ciesielski and Sterckeman1997), to be 95 meq g–1 and the basal spacing was also determined by Belaidi et al. (Reference Belaidi, Bedrane, Choukchou-Braham and Bachir2015) by XRD to be 13.8 Å. To produce the acid-activated clay, referred to as AAC, the initial bentonite was dispersed in a 1.0 M HCl solution (Biochem Chemopharma, 37%, France) and stirred vigorously at 80°C for 4 h. The resulting AAC material was then modified by the incorporation of Ti polycations as pillars, following the procedure of Yamanka et al. (Reference Yamanka, Nishihara and Hattori1987). Following incorporation of Ti, the solid was separated by centrifugation using a Rotofix 32A Hettich centrifuge (Andreas Hettich, Germany) at (6000 rpm, 4226×g) and subsequently dried at 80°C for 16 h. To obtain Ti-pillared, acid-activated clay, denoted as Ti-AAC, the material was finally calcined at 400°C for 3 h. The method of Bahranowski et al. (Reference Bahranowski, Dula, Komorek, Romotowski, Serwicka, Poncelet, Martens, Delmon, Jacobs and Grange1995) was followed to prepare the material with 5% mass of vanadium. Then, it was dried at 80°C for 20 h and calcined at 500°C for 5 h. The catalyst prepared was referred to thereafter as 5V/Ti-AAC.
Characterization methods
The 5V/Ti-AAC samples were characterized using various techniques. X-ray powder analysis (XRD) was performed using a Rigaku D/max 2500 diffractometer (Rigaku Corporation, Japan) using Ni-filtered CuKα radiation (λ = 1.541874 Å). The scanning range was between 2.5 and 70°2θ at a scanning speed of 0.03°2θ/s. Prior to analysis the samples were ground to fine powder. N2 adsorption-desorption analysis was conducted using a Quantachrome Nova 1000e instrument (Quantachrome Corporation, France) to determine the specific surface area, pore volume, and micropore volume. The pore-size distribution was calculated using the Barret-Joyner-Halenda (BJH) method for the desorption branch (Bardestani et al., Reference Bardestani, Patience and Kaliaguine2019), and the micropore volume and micropore surface area were calculated by the t-plot-de Boer method (Lippens and de Boer, Reference Lippens and De Boer1995). Prior to physisorption measurements, the samples were outgassed at 250°C for 3 h under vacuum.
Diffuse-reflectance UV-visible (UV-Vis) spectra in the range 200–800 nm were obtained using a Perkin-Elmer Lambda 800 UV-Vis spectrometer (PerkinElmer, Massachusetts, USA). The samples in powder form were placed in the sample holder and the baseline was established using BaSO4 as a reference. Fourier-transform infrared spectroscopy (FTIR) analysis was conducted at room temperature using a Cary 600 FTIR spectrometer (Agilent Technologies, California, USA). The modified bentonite materials were dried in a hot air oven at 100°C for 1 h before pyridine treatment in order to remove moisture. Then, the samples were placed in direct contact with pyridine (Aldrich, 99.8%). Prior to FTIR measurements the samples were heated in an oven at 120°C for 1 h in order to remove the physisorbed pyridine. The FTIR spectra were recorded over the spectral range 1650–1350 cm–1 at a resolution of 4 cm–1, using KBr as a background. Finally, the scanning electron microscopy (SEM) with energy-dispersive X-ray elemental analysis (EDX) was performed using a Hitachi-TM1000 instrument (Elexience, France) at an acceleration voltage of 150 kV.
Catalytic experiments
The catalytic epoxidation of cyclohexene (Labosi, 99%, France) was carried out using tert-butyl hydroperoxide, TBHP (Sigma Aldrich, 70 wt.% in H2O, France), as the oxidant in a two-neck, glass, round-bottom flask equipped with a magnetic stirrer and reflux condenser. Initially, 20 mL of solvent and 38.45 mmol (5.5 mL) of TBHP were combined in a closed Erlenmeyer flask and stirred magnetically for 24 h. Afterward, the organic phase was separated from the aqueous phase. Subsequently, 29 mmol (3 mL) of cyclohexene was added to the TBHP-solvent mixture. The reaction mixture was heated to 65°C with vigorous stirring, and then the catalyst (100 mg) was introduced at time zero. The progress of the reaction was monitored using gas chromatography (GC) with a Shimadzu GC 14-B instrument (Shimadzu, France) equipped with an Agilent HP-FFAP capillary column and a flame ionization detector (FID). The remaining TBHP was determined by iodometric titration of the organic phase at the end of the reaction.
where TOF = turnover frequency.
Results and discussion
Materials characterization
The XRD patterns of the AAC, Ti-AAC, and 5V/Ti-AAC samples (Fig. 1) revealed the d 001 basal spacing for AAC at 6°2θ to be 14.7 Å (Table 1), indicating that the periodic structure of the 2:1 layers remained largely intact following the acid treatment. (Bailey et al., Reference Bailey, Brindley and Brown1980). On the other hand, this basal spacing was absent from the XRD pattern for the Ti-AAC pillared acid-activated clay (Table 1), indicating that the layer structure was less well ordered. Clearly, the intercalation process by the small, hydrolyzed Ti moiety yielded non-uniform interlayer distances or perhaps even a delaminated structure (Chen et al., Reference Chen, Zhu, Zhou, Deng and Li2012; Yuan et al., Reference Yuan, Yin, He, Yang, Wang and Zhu2006).

Figure 1. XRD patterns of (a) AAC, (b) Ti-AAC, and (c) 5V/Ti-AAC.
Table 1. Basal spacing of based clay materials

The XRD pattern for the Ti-AAC also revealed a reflection at 19–20°2θ attributed to the sum of the indices hk (02) and (11), and that at 35°2θ to the sum of the indices hk (13) and (20). The reflection at 25.3°2θ corresponds to TiO2 anatase (Bineesh et al., Reference Bineesh, Kim and Park2010b; Chaker et al., Reference Chaker, Fourmentin and Chérif-Aouali2020; Long and Yang, Reference Long and Yang2000b).
The XRD data for 5V/Ti-AAC are similar to those of Ti-AAC. No reflection representing vanadium oxide was observed; vanadium oxide appears only if the vanadium loading exceeds 15% according to Long and Yang (Reference Long and Yang2000b).
The adsorption and the adsorption-desorption isotherms of the AAC, Ti-AAC, and 5V/Ti-AAC (Fig. 2) are of type IV (IUPAC classification), which are indicative of mesoporous materials (Azzi et al., Reference Azzi, Bendahou, Cherif-Aouali, Hamidi, Siffert, Bengueddach and Aboukais2012, Reference Azzi, Chérif and Siffert2020). Moreover, during the pillar formation of Ti-AAC (Fig. 2), the slope of the adsorption-desorption isotherm increased at relatively low pressures. The materials investigated exhibited a monomodal pore-size distribution, with an average diameter ranging from 3.6 to 3.8 nm (Fig. 2). The textural properties of the prepared materials, including BET specific surface area, pore volume, micropore volume, and pore diameter, are summarized in Table 2. An increase in specific surface area was observed, from 196 to 237 m2 g–1 after Ti intercalation. This enhancement in specific surface area is attributed to the successful formation of pillars (Bineesh et al., Reference Bineesh, Cho, Kim, Jermy and Park2008, Reference Bineesh, Kim, Jermy and Park2009).

Figure 2. N2 adsorption-desorption isotherms and BJH pore-size distribution.
Table 2. Properties of the materials
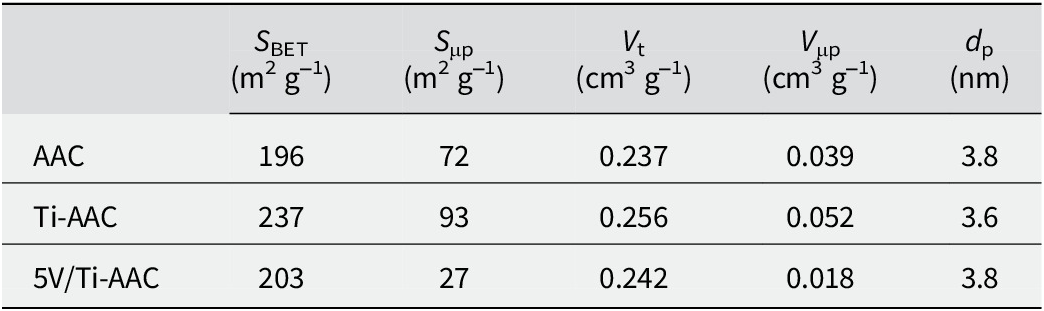
S μp: micropore surface area; V t: total pore volume; V μp: micropore volume; d p: pore diameter.
The considerable specific surface area of Ti-AAC facilitates the effective dispersion of vanadium. However, upon impregnation of vanadium species, a reduction in specific surface area was observed. This decrease was attributed to the partial blockage of mesopores caused by the presence of vanadium species (Azzi et al., Reference Azzi, Bendahou, Cherif-Aouali, Hamidi, Siffert, Bengueddach and Aboukais2012, Reference Azzi, Chérif and Siffert2020; Bineesh et al., Reference Bineesh, Cho, Kim, Jermy and Park2008, Reference Bineesh, Kim, Jermy and Park2009). Furthermore, a decline in the volume of micropores was observed following the impregnation of vanadium, probably resulting from the partial occlusion of Ti-AAC micropores by vanadium oxides. This phenomenon was similarly documented in the study conducted by Arfaoui et al. (Reference Arfaoui, Khafallah Boudali and Ghorbel2006).
The DR UV-Vis spectrum of the AAC sample (Fig. 3) exhibited a broad band centered at ~250 nm which is assigned to charge transfer at the octahedral iron sites (Fe3+←O2–, OH– or OH2) of montmorillonite in the bentonite (Arfaoui et al., Reference Arfaoui, Boudali, Ghorbel and Delahay2009).

Figure 3. DR UV-vis spectra of (a) AAC, (b)Ti-AAC, and (c) 5V/Ti-AAC.
The DR UV-Vis of Ti-AAC displayed two main bands, one at 250 nm, which carried over from AAC, and the other at ~300 nm (Fig. 3), which may be due to charge transfer from the valence band (mainly formed by 2p orbitals of the oxide anion) to the conduction band (mainly formed by 3d t2g orbitals of the Ti4+ cations) (Bineesh et al., Reference Bineesh, Kim, Kim and Park2011). This band is consistent with Ti occurring in the form of anatase (Bineesh et al., Reference Bineesh, Kim, Kim, Cho and Park2010a, Reference Bineesh, Kim, Kim and Park2011).
The 5V/Ti-AAC spectrum showed three charge-transfer (CT) bands of V5+: the first at ~270 nm, the second at ~350 nm, and the third at 390 nm. The band at 270 nm was assigned to the low-energy ligand-to-metal (O2− to V5+) charge-transfer (LMCT) transitions associated with the isolated tetrahedral monomeric V species (Concepcion et al., Reference Concepcion, Navarro, Blasco, Lopeznieto, Panzacchi and Rey2004; Iannazzo et al., Reference Iannazzo, Neri, Galvagno, Di Serio, Tesser and santacesaria2003). The band at 350 nm was assigned to the weak chains of polymerized V involving both tetrahedral and square pyramidal configurations of V (Concepcion et al., Reference Concepcion, Navarro, Blasco, Lopeznieto, Panzacchi and Rey2004; El-Korso et al., Reference El-Korso, Bedrane, Choukchou-Braham and Bachir2015; Iannazzo et al., Reference Iannazzo, Neri, Galvagno, Di Serio, Tesser and santacesaria2003). The band at 390 nm was assigned to (O2− to V5+) charge-transfer transitions associated with the isolated octahedral monomeric species (Chary et al., Reference Chary, Kumar, Naresh, Bhaskar and Sakata2006; Held et al., Reference Held, Kowalska-Kuś and Nowińska2012; Murgia et al., Reference Murgia, Torres, Gottifredi and Sham2006).
The deposition or impregnation of vanadium into Ti-AAC caused a slight decrease in the main absorption intensity of the bands at 270 nm and 350 nm, which results from the disruption of the Ti-O-Ti chain by the incorporation of the vanadium species (Bineesh et al., Reference Bineesh, Kim, Kim, Cho and Park2010a, Reference Bineesh, Kim, Kim and Park2011).
The FTIR spectra over the range 1340–1640 cm–1 (Fig. 4) of AAC, Ti-AAC, and 5V/Ti-AAC materials before and after pyridine adsorption revealed part of a broad band at ~1640 cm–1, which was assumed to be from H-O-H bending vibrations from adsorbed water. Prior to pyridine treatment, no significant absorption bands were evident except for the OH groups. To remove physisorbed pyridine, the pyridine-treated samples were heated at 120°C for 1 h. No color change was observed in the materials upon pyridine treatment or after heat treatment. The FTIR spectrum of AAC displayed two distinctive bands at 1545 and 1490 cm–1, corresponding to the acidic sites of Brønsted and Brønsted/Lewis, respectively (Azzi et al., Reference Azzi, Rekkab-Hammoumraoui, Chérif-Aouli and Choukchou-Braham2019; Candan Karaeyvaz and Balci, Reference Candan Karaeyvaz and Balci2021). The Ti-AAC spectrum shows four characteristic bands at 1545, 1515, 1490, and 1448 cm-1, corresponding to the acidic sites of Brønsted, Brønsted/Lewis, and Lewis, respectively. In addition, the Ti-AAC spectrum shows the appearance of two new bands at 1515 and 1448 cm–1 compared to that of AAC, which are characteristic of the acidity provided by the titanium hydroxides inserted into acidified bentonite matrix. The 5V/Ti-AAC material still shows the presence of the four bands, indicating the Brønsted, Brønsted/Lewis, and Lewis acid sites.

Figure 4. FTIR spectra (a) before and (b) after pyridine adsorption.
The Brønsted and Lewis acid sites were estimated by calculating the area of the Peakfit absorption bands, then these areas were used to quantify the acid sites (Table 3). These results show that the acid-activated bentonite exhibits a Brønsted acidity which decreased after intercalation of the titanium, although the Lewis acidity does not appear until after the introduction of the titanium. After impregnation with vanadium, a slight decrease in Brønsted acidity was recorded with the reappearance of the four acidity bands.
Table 3. Lewis and Brønsted acid sites accessible to pyridine used as probe molecules estimated by the FTIR integrated bands area

The morphology of various samples examined via SEM coupled with EDX (Fig. 5) found large particle aggregates with smooth surfaces in the AAC sample, while the 5V/Ti-AAC sample showed the formation of disordered structures at the end of the delamination process, in accordance with XRD results. The EDX results confirmed the presence of Ti and V after intercalation.

Figure 5. SEM images and EDX analysis of (a) AAC and (b) 5V/Ti-AAC.
Catalytic activity
Here the oxidation of cyclohexene proceeds through two distinct pathways. The first pathway involves a direct epoxidation process facilitated by redox catalysts, leading to the formation of the epoxide product. On the other hand, the second pathway involves an allylic oxidation mechanism in the presence of Lewis acid catalysts, resulting in the formation of cyclohexenol, cyclohexenone, cyclohexanone, and cyclohexanol as products (Azzi et al., Reference Azzi, Rekkab-Hammoumraoui, Chérif-Aouli and Choukchou-Braham2019). As is well known, the VO x active species have a significant impact on the catalytic performance of vanadium-supported catalysts due to the presence of surface redox as well as acid sites (El-Korso et al., Reference El-Korso, Khaldi, Bedrane, Choukchou-Braham, Thibault-Starzyk and Bachir2014).
Initially, the catalytic performance of the 5V/Ti-AAC catalyst was investigated in the epoxidation reaction of cyclohexene, using tert-butyl hydroperoxide TBHP as the oxidant, and heptane and acetonitrile as solvents. The conversion, selectivity, and TOF of the studied reaction are calculated using equations 1, 2, and 3 respectively. The results obtained are presented in Table 4. Both the support AAC and Ti-AAC exhibited low activity toward cyclohexene epoxidation, with minimal TBHP consumption. However, the impregnation of Ti-AAC with vanadium resulted in excellent catalytic activity, primarily yielding the epoxide as the major product. This enhancement in catalytic performance can be attributed to the presence of weak acid sites (Table 4) and the strong interaction between vanadium and titanium in the 5V/Ti-AAC catalyst (Comite et al., Reference Comite, Sorrentino, Capannelli, Di Serio, Tesser and Santacesaria2003; El-Korso et al., Reference El-Korso, Khaldi, Bedrane, Choukchou-Braham, Thibault-Starzyk and Bachir2014). It can also be explained by the high redox potentials of Ti4+/Ti3+ (2.5 V) and V5+/V4+ (4.5 V) couples (Nanjundaswamy et al., Reference Nanjundaswamy, Padhi, Goodenough, Okadab, Ohtsukab, Araib and Yamaki1996; Patoux and Masquelier, Reference Patoux and Masquelier2002).
Table 4. Epoxidation of cyclohexene using different solvents
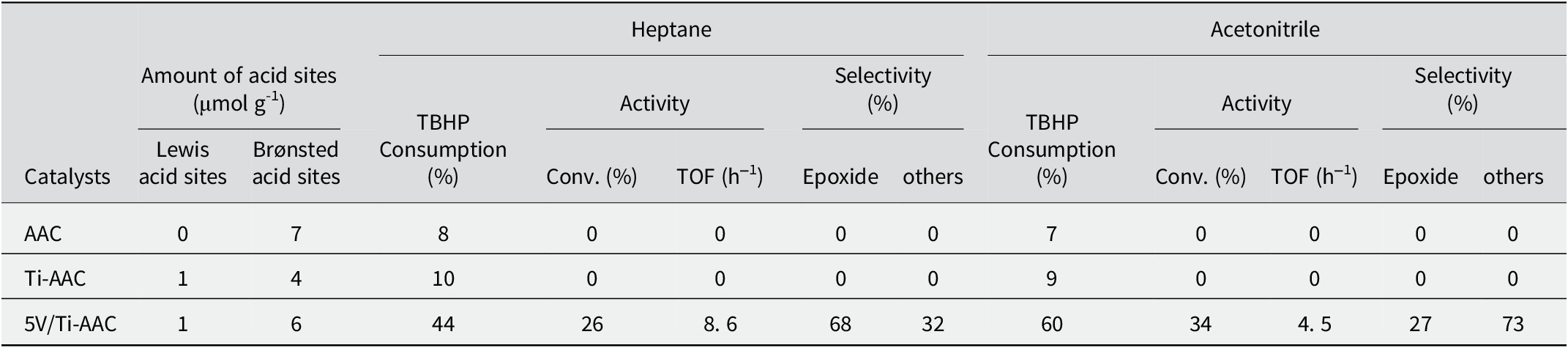
Reaction conditions. cyclohexene 29 mmol, TBHP 38.45 mmol, solvent 25 mL, 0.1 g catalyst, 6 h, reaction temperature 65°C.
Moreover, it was observed that the use of acetonitrile as the solvent led to a greater conversion of cyclohexene (34%) compared to heptane (26%). However, this change in the solvent resulted in a significant decrease in the selectivity of cyclohexene epoxide, with almost half of the desired product being lost. These results suggest that acetonitrile tends to favor the oxidation pathway rather than the epoxidation pathway, possibly due to the solvent covering the intermediate sites formed between TBHP and vanadium oxide. Similar observations were reported by Ouidri et al. (Reference Ouidri, Guillard, Caps and Khalaf2010). This phenomenon can be attributed to the hydrophobic nature of the clay intercalated by titanium. The support exhibits a strong affinity for cyclohexene due to the reduction in polarity on its surface caused by the intercalation process, resulting in the generation of a hydrophobic surface (Shimizu et al., Reference Shimizu, Kaneko, Fujishima, Kodama, Yoshida and Kitayama2002). The polar solvent, acetonitrile, serves as a transporter, facilitating the entry of cyclohexene molecules from the external environment into the catalyst pores. Within these pores, the oxidation reaction occurs on active catalytic sites containing one or more vanadium atoms. Conversely, in the case of heptane, the affinity for the materials prepared is considerably weaker due to its lower polarity and uncharged nature. These factors collectively account for the greater conversion rates observed with acetonitrile compared to heptane. Acetonitrile’s polar nature promotes a stronger interaction with the catalytic support, leading to changes in the size and shape of the pores. Consequently, this alteration results in a reduced selectivity toward the epoxidation reaction. To address this concern, heptane is employed as the solvent for subsequent studies. The use of heptane as a solvent helps to maintain the desired selectivity while maintaining acceptable conversion rates.
Based on the findings presented in Table 5, the quantity of catalyst significantly impacts the conversion of cyclohexene, with values ranging from 26% to 18% as the catalyst mass varied from 100 mg to 30 mg. This variation could be attributed to the reduction in active vanadium sites at lower catalyst masses. Conversely, a slight improvement in epoxide selectivity was observed when using a 30 mg 5V/Ti-AAC catalyst. Furthermore, increasing the catalyst amount to 150 mg led to a substantial increase in cyclohexene conversion from 26% to 46%. However, it also resulted in a sharp decline in epoxide selectivity from 68% to 39%. This phenomenon is explained by the synergistic effect of vanadium sites acting as active sites, which intensifies with greater catalyst mass. To strike a balance between conversion and selectivity, the subsequent study will be conducted using 100 mg of catalyst.
Table 5. Effect of the amount of catalyst on the cyclohexene epoxidation

Reaction conditions. Cyclohexene 29 mmol, TBHP 38.45 mmol, Heptane 25 mL, 6 h, reaction temperature 65°C.
The cyclohexene epoxide selectivity is also influenced by the quantity of TBHP which is another important parameter. To explore this, the effect of the amount of TBHP was examined while keeping the cyclohexene substrate constant. Various cyclohexene to TBHP molar ratios were tested, ranging from 1.3 to 3, using 100 mg of 5V/Ti-AAC catalyst. As indicated in Table 6, increasing the amount of oxidant does not have a significant impact on the selectivity of reaction products; the selectivity remained unchanged for cyclohexene epoxide and underwent minor changes for other byproducts. This finding aligns with a similar result reported by Kumaresan et al. (Reference Kumaresan, Prabhu, Palanichamy and Murugesan2010). Conversely, an elevation in the TBHP/cyclohexene molar ratio led to a direct increase in cyclohexene conversion. This phenomenon can be attributed to the alteration of the hydrophilic/hydrophobic balance in the reaction medium caused by the addition of TBHP, which possesses a hydrophilic nature. Based on the catalytic experimental results and in line with findings from various literature sources, the observed increase in cyclohexene conversion can be attributed to the change in the solvent environment due to the hydrophilic nature of TBHP (Farzaneh et al., Reference Farzaneh, Zamanifar and Williams2004; Sedighipoor et al., Reference Sedighipoor, Kianfar, Mahmood and Azarian2017; Su et al., Reference Su, Li, Huo, Guan and Kan2014).
Table 6. Effect of TBHP/Cyclohexene molar ratio on the cyclohexene epoxidation
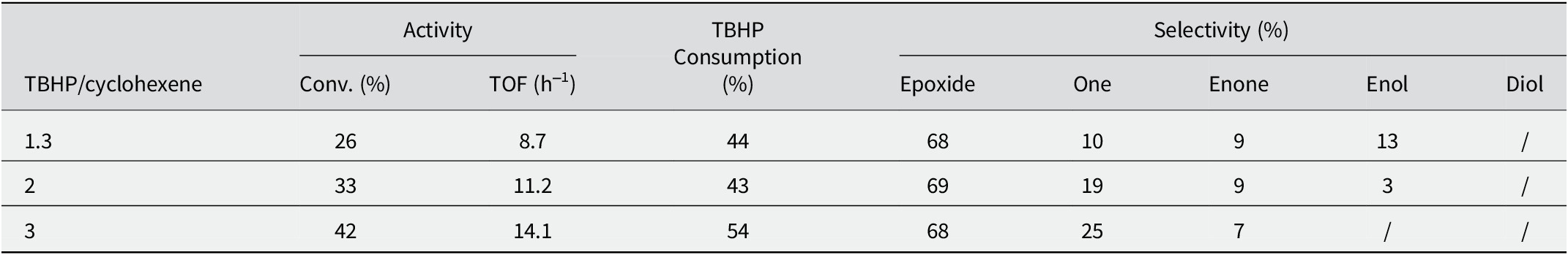
Reaction conditions: cyclohexene 29 mmol, Heptane 25 mL, 0.1 g catalyst, 6 h, reaction temperature 65°C.
The proposed mechanism for the reaction (Fig. 6) reveals that the vanadium (+V) forms a complex with TBHP, resulting in an intermediate metal peroxide complex. Subsequently, cyclohexene interacts with one of the metal-peroxo bonds, leading to the formation of the peroxo metallocycle. The peroxo-metallocycle undergoes a breakage process, generating cyclohexene oxide, while the 5V/Ti-AAC catalyst is regenerated to initiate a new reaction cycle. Furthermore, the catalytic performance of the 5V/Ti-AAC catalyst was compared to that reported in the literature for cyclohexene epoxidation. The results demonstrate that the 5V/Ti-AAC material ranks among the most active catalysts (Table 7).

Figure 6. Proposed mechanism of epoxidation of cyclohexene over the 5V/Ti-AAC catalyst.
Table 7. Comparison of the present work with literature

The variation of cyclohexene conversion with reaction time at three different temperatures (60°C, 70°C, and 80°C) is illustrated in Fig. 7. The present kinetic study aimed to determine the activation energy of the epoxidation reaction of cyclohexene using the 5V/Ti-AAC catalyst. The reaction conditions included a TBHP/Cyclohexene molar ratio of 3 and a catalyst mass of 100 mg. Furthermore, the decrease in selectivity toward cyclohexene epoxide with increasing reaction temperature is noted (Fig. 8).

Figure 7. Epoxidation reaction of cyclohexene as a function of temperature.

Figure 8. Epoxide selectivity as function of reaction temperature.
The rate of the epoxidation reaction of cyclohexene is written as follows:
and
where α is partial order related to cyclohexene; β is partial order related to TBHP; t is time of reaction; and k is the rate constant of the reaction.
In order to discover reaction order, α, the authors assumed
![]() $ {\left[\mathrm{TBHP}\right]}^{\unicode{x03B2}} $
to be constant because it is in excess, therefore:
$ {\left[\mathrm{TBHP}\right]}^{\unicode{x03B2}} $
to be constant because it is in excess, therefore:
Then:
where k
![]() $ ^{\prime } $
is the rate constant of the reaction.
$ ^{\prime } $
is the rate constant of the reaction.
In the present work, the reaction was assumed to have an order of 2 related to cyclohexene. For that reason, the integration of the equation 7 is:
where C is the cyclohexene concentration at given time t; and C 0 is the initial cyclohexene concentration.
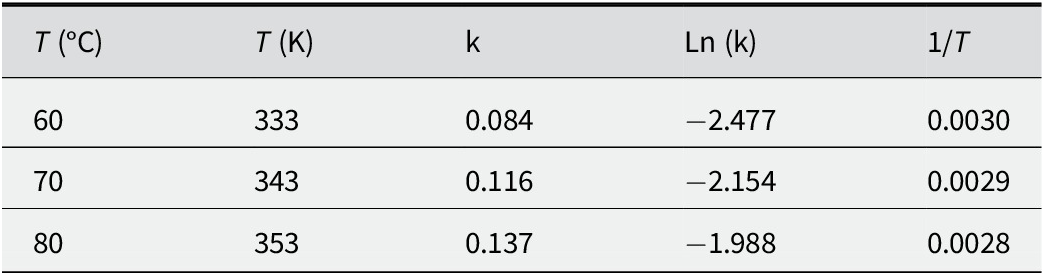
To establish the kinetic order of the cyclohexene epoxidation reaction over the 5V/Ti-AAC catalyst, the reciprocal of concentration (1/C) was plotted against the reaction time at various reaction temperatures. The linearity of each equation (Fig. 9), proves that the reaction follows a kinetic order of 2.

Figure 9. Kinetic data at various temperatures for the epoxidation of cyclohexene.
R 2 coefficients are ~1 for the three plots and are straight lines which prove that the reaction of epoxidation of cyclohexene has a kinetic order of 2. The calculated rate constant for each reaction temperature test is shown in Table 8.
Table 8. Rate constant value as a function of reaction temperature
According to the Arrhenius equation:
where k is the activation energy, A is the pre-exponential factor, E a is the activation energy, R is the universal gas constant and T is the absolute temperature.
To calculate activation energy, –ln(k) was plotted versus 1/T (Fig. 10).

Figure 10. Arrhenius plot of the epoxidation of cyclohexene.
According to the tendency curve we have:
![]() $ \frac{E_{\mathrm{a}}}{\mathrm{R}}=2883 $
with R = 8.314 kJ kmol–1 K–1. So, the value of the activation energy will be:
$ \frac{E_{\mathrm{a}}}{\mathrm{R}}=2883 $
with R = 8.314 kJ kmol–1 K–1. So, the value of the activation energy will be:
The activation energy of the cyclohexene epoxidation over 5V/Ti-AAC is among the lowest energy in the literature (Kwon et al., Reference Kwon, Schweitzer, Park, Stair and Snurr2015).
The stability of the catalyst is a crucial parameter that determines its potential for multiple reuses in consecutive reaction runs under identical conditions. To investigate the stability of the 5V/Ti-AAC catalyst in the cyclohexene epoxidation reaction, we conducted reusability tests for three reaction runs. After each run, the catalyst was separated from the reaction mixture via simple filtration. Subsequently, the catalyst was washed multiple times with the reaction solvent and dried at room temperature to prepare it for the next reaction run. The results of the catalyst reusability tests (Table 9) indicate a slight decrease in both reaction conversion and epoxide selectivity after three reaction runs. This observation can be attributed to the loss of catalyst during the recovery process after each reaction run. Despite the slight reduction in performance, the catalyst demonstrated promising reusability potential for multiple reaction cycles.
Table 9. Catalyst stability reaction tests
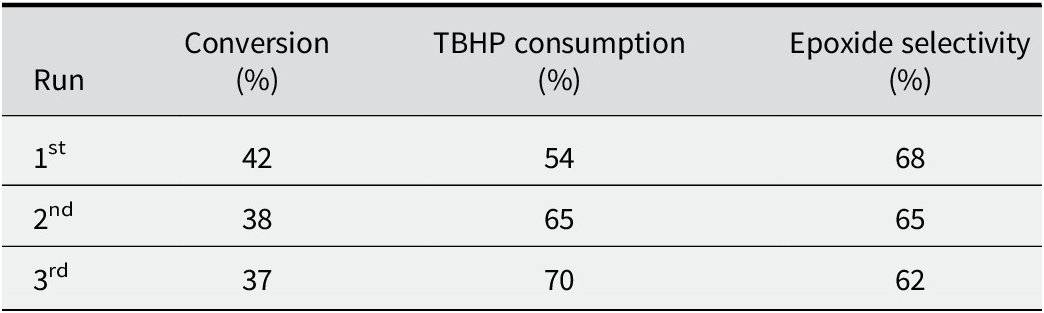
The recovered reaction mixtures were tested under the same reaction conditions by adding a quantity of cyclohexene in order to determine the vanadium leaching. The result shows no production of epoxide or byproducts as found by Brutchey et al. (Reference Brutchey, Mork, Sirbuly, Yang and Tilley2005) and Farzaneh et al. (Reference Farzaneh, Zamanifar and Williams2004).
Summary and conclusions
This investigation focused on exploring the potential of vanadium oxide-supported acid-modified clay (Ti-AAC) materials as catalysts for the environmentally friendly cyclohexene epoxidation reaction. The synthesized 5V/Ti-AAC material exhibited a mesoporous structure with introduced Lewis acidity through the incorporation of titanium into the clay matrix. Detailed analysis, including DR UV-vis, confirmed the presence of octahedral titanium in the anatase form and the existence of vanadium (+V) in the form of polymeric species V-O-V. The catalytic performance of 5V/Ti-AAC was exceptional, achieving a cyclohexene conversion of 42% with an impressive epoxide selectivity of 68%. These encouraging results were achieved under specific conditions, including a TBHP/cyclohexene molar ratio of 3, 100 mg of catalyst, and a reaction temperature of 65°C. Notably, the redox centers in the catalyst played a crucial role in directing the reaction toward epoxidation, surpassing the contribution of acid sites. The kinetic study revealed that the cyclohexene epoxidation reaction followed a second-order reaction with activation energy of 23.97 kJ mol–1. This value surpassed various reported activation energies in previous research, indicating the catalyst’s efficiency and potential for industrial applications. In addition, the reusability of the 5V/Ti-AAC catalyst demonstrated remarkable stability. It maintained its catalytic efficacy throughout three consecutive reaction runs, showcasing the potential for multiple cycles without significant loss in epoxide selectivity. Finally, this study developed successfully a highly efficient, environmentally friendly, and reusable 5V/Ti-AAC catalyst for the cyclohexene epoxidation reaction. The catalyst’s excellent performance and stability make it a promising candidate for sustainable and economically viable epoxidation processes in industrial applications.
Acknowledgments
The authors are grateful for the XRD analysis done at the laboratory for Heterogeneous Catalysis for Selective Organic Synthesis at the Institute of Chemical Synthesis and Homogeneous Catalysis of the University of Zaragoza-CSIC, Spain.
Financial support
The authors thank the General Directorate for Scientific Research and Technological Development (DGRSDT) and the Thematic Research Agency of Science and Technology (ATRST), Algeria, for financial support.
Competing interests
The authors declare no competing interests.






















