Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Heiberg, Lisa
Pedersen, Thomas Vils
Jensen, Henning S.
Kjaergaard, Charlotte
and
Hansen, Hans Christian Bruun
2010.
A Comparative Study of Phosphate Sorption in Lowland Soils under Oxic and Anoxic Conditions.
Journal of Environmental Quality,
Vol. 39,
Issue. 2,
p.
734.
Davesne, E.
Dideriksen, K.
Christiansen, B.C.
Sonne, M.
Ayala-Luis, K.B.
Koch, C. Bender
Hansen, H.C.B.
and
Stipp, S.L.S.
2010.
Free energy of formation for green rust sodium sulphate (NaFeII6FeIII3(OH)18(SO4)2(s)).
Geochimica et Cosmochimica Acta,
Vol. 74,
Issue. 22,
p.
6451.
Bruggeman, C.
Maes, N.
Christiansen, B.C.
Stipp, S.L.S.
Breynaert, E.
Maes, A.
Regenspurg, S.
Malström, M.E.
Liu, X.
Grambow, B.
and
Schäfer, Th.
2012.
Redox-active phases and radionuclide equilibrium valence state in subsurface environments – New insights from 6th EC FP IP FUNMIG.
Applied Geochemistry,
Vol. 27,
Issue. 2,
p.
404.
Powell, Daniel
Fincher, Dale
Gonzales, Raymond
Melancon, Burkin
Winters, Robert H.
Rodrigue, Brad
and
Brown, Elliot
2012.
Failure Analysis: Initial Corrosion Products Protect a Pipeline from Microbiologically Generated H2S.
Materials Performance,
Vol. 51,
Issue. 8,
p.
66.
Frau, Franco
Medas, Daniela
Da Pelo, Stefania
Wanty, Richard B.
and
Cidu, Rosa
2015.
Environmental Effects on the Aquatic System and Metal Discharge to the Mediterranean Sea from a Near-Neutral Zinc-Ferrous Sulfate Mine Drainage.
Water, Air, & Soil Pollution,
Vol. 226,
Issue. 3,
Sun, Jing
Chillrud, Steven N.
Mailloux, Brian J.
Stute, Martin
Singh, Rajesh
Dong, Hailiang
Lepre, Christopher J.
and
Bostick, Benjamin C.
2016.
Enhanced and stabilized arsenic retention in microcosms through the microbial oxidation of ferrous iron by nitrate.
Chemosphere,
Vol. 144,
Issue. ,
p.
1106.
Channei, Duangdao
Phanichphant, Sukon
Nakaruk, Auppatham
Mofarah, Sajjad
Koshy, Pramod
and
Sorrell, Charles
2017.
Aqueous and Surface Chemistries of Photocatalytic Fe-Doped CeO2 Nanoparticles.
Catalysts,
Vol. 7,
Issue. 2,
p.
45.
Usman, M.
Byrne, J. M.
Chaudhary, A.
Orsetti, S.
Hanna, K.
Ruby, C.
Kappler, A.
and
Haderlein, S. B.
2018.
Magnetite and Green Rust: Synthesis, Properties, and Environmental Applications of Mixed-Valent Iron Minerals.
Chemical Reviews,
Vol. 118,
Issue. 7,
p.
3251.
Su, Chunming
and
Wilkin, Richard T.
2020.
Removal of Arsenate and Arsenite in Equimolar Ferrous and Ferric Sulfate Solutions through Mineral Coprecipitation: Formation of Sulfate Green Rust, Goethite, and Lepidocrocite.
Soil Systems,
Vol. 4,
Issue. 4,
p.
68.
Doggaz, Amira
Coustel, Romain
Durand, Pierrick
Humbert, François
and
Ruby, Christian
2020.
Birnessite: A New Oxidant for Green Rust Formation.
Materials,
Vol. 13,
Issue. 17,
p.
3777.
Doi, Minori
Harada, Makoto
and
Okada, Tetsuo
2021.
Freezing Enhances Leaching of Ferrous Ions but Hinders Reductive Dissolution of Ferric Ions from Iron Oxides.
ACS Earth and Space Chemistry,
Vol. 5,
Issue. 6,
p.
1544.
Cornu, Damien
Coustel, Romain
Renaudin, Guillaume
Rogez, Guillaume
Renard, Aurélien
Durand, Pierrick
Carteret, Cédric
and
Ruby, Christian
2022.
Synthesis and characterization of a new monometallic layered double hydroxide using manganese.
Dalton Transactions,
Vol. 51,
Issue. 31,
p.
11787.
Wang, Danqian
Yue, Yanfei
Xie, Zhichao
Mi, Tangwei
Yang, Siyu
McCague, Colum
Qian, Jueshi
and
Bai, Yun
2022.
Chloride-induced depassivation and corrosion of mild steel in magnesium potassium phosphate cement.
Corrosion Science,
Vol. 206,
Issue. ,
p.
110482.
Ai, Jing
Chr. Bruun Hansen, Hans
Dideriksen, Knud
and
Tobler, Dominique J.
2022.
Fine-tuning green rust − bone char composite synthesis for efficient chlorinated ethylene remediation.
Chemical Engineering Journal,
Vol. 446,
Issue. ,
p.
136770.
Tamisier, Marc
Musat, Florin
Richnow, Hans-Hermann
Vogt, Carsten
and
Schmidt, Matthias
2023.
On the corrosion of ductile cast iron by sulphate reducing bacteria—implications for long-term nuclear waste repositories.
Frontiers in Geochemistry,
Vol. 1,
Issue. ,
Alam, Khondaker M. N.
and
Elzinga, Evert J.
2023.
Dynamics and Mechanisms of Mn(II), Co(II), Ni(II), Zn(II), and Cd(II) Sorption onto Green Rust Sulfate.
Environmental Science & Technology,
Vol. 57,
Issue. 22,
p.
8396.
Li, Wei-Zheng
Mahasti, Nicolaus N.N.
Chang, Kai-Yang
and
Huang, Yao-Hui
2023.
Application of Fe0.66Cu0.33@Al(OH)3 catalyst from fluidized-bed crystallizer by-product for RB5 azo dye treatment using visible light-assisted photo-Fenton technology.
Chemosphere,
Vol. 343,
Issue. ,
p.
140268.
Wang, Xin
Wells, Naomi S.
Xiao, Wei
Hamilton, Jessica L.
Jones, Adele M.
and
Collins, Richard N.
2023.
Abiotic reduction of nitrate to ammonium by iron (oxy)(hydr)oxides and its stable isotope (δ15N, δ18O) dynamics.
Geochimica et Cosmochimica Acta,
Vol. 347,
Issue. ,
p.
28.
Lv, Ying
Han, Linting
Shen, Jie
Li, Jianfa
Dong, Huaping
Hu, Jiangang
He, Yuxin
and
Li, Yimin
2024.
Impact of environmental factors on the removal of chloramphenicol by zero-valent iron and pyrite mixture.
Separation and Purification Technology,
Vol. 329,
Issue. ,
p.
125045.
Vital, Melanie
van Beek Pedersen, Theis
Molander, Jakob
Jakobsen, Rasmus
Tobler, Dominique J.
and
Dideriksen, Knud
2025.
Dissolution kinetics for the Fe(II)-Fe(III) layered double hydroxide, green rust.
Applied Clay Science,
Vol. 272,
Issue. ,
p.
107814.
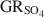 ) with the formation of magnetite was used as a novel method to determine the standard Gibbs energy of formation of GRSO4\$\end{document}
) with the formation of magnetite was used as a novel method to determine the standard Gibbs energy of formation of GRSO4\$\end{document}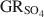 , ΔfGo(GRSO4)\$\end{document}
, ΔfGo(GRSO4)\$\end{document}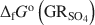 . Aqueous suspensions of GRSO4\$\end{document}
. Aqueous suspensions of GRSO4\$\end{document}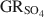 , with pH slightly >8, were titrated slowly with 1 M H2SO4 until pH = 3 under strict anoxic conditions. Powder X-ray diffraction and Mössbauer analysis revealed that magnetite was the only solid phase formed during the initial part of the titration, where the equilibrium pH was maintained above 7.0. The ratio of Fe2+ release to consumption of protons confirmed the stoichiometry of dissolution of GRSO4\$\end{document}
, with pH slightly >8, were titrated slowly with 1 M H2SO4 until pH = 3 under strict anoxic conditions. Powder X-ray diffraction and Mössbauer analysis revealed that magnetite was the only solid phase formed during the initial part of the titration, where the equilibrium pH was maintained above 7.0. The ratio of Fe2+ release to consumption of protons confirmed the stoichiometry of dissolution of GRSO4\$\end{document}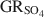 and the formation of magnetite at equilibrium conditions. The estimate of the absolute value of ΔfGo(GRSO4)\$\end{document}
and the formation of magnetite at equilibrium conditions. The estimate of the absolute value of ΔfGo(GRSO4)\$\end{document}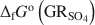 was −3819.43±6.44 kJ mol−1 + y × [ΔfGo(H2O(1))], where y is the number of interlayer water molecules per formula unit. The logarithm of the solubility product, log Ksp, was estimated to be −139.2±4.8 and is invariable with y. Using the new value for ΔfGo(GRSO4)\$\end{document}
was −3819.43±6.44 kJ mol−1 + y × [ΔfGo(H2O(1))], where y is the number of interlayer water molecules per formula unit. The logarithm of the solubility product, log Ksp, was estimated to be −139.2±4.8 and is invariable with y. Using the new value for ΔfGo(GRSO4)\$\end{document}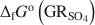 , the reduction potentials of several GRSO4\$\end{document}
, the reduction potentials of several GRSO4\$\end{document}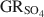 -Fe oxide couples were evaluated, with the GRSO4\$\end{document}
-Fe oxide couples were evaluated, with the GRSO4\$\end{document}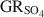 -magnetite half cell showing the smallest redox potential at pH 7 and free ion activities of 10−3.
-magnetite half cell showing the smallest redox potential at pH 7 and free ion activities of 10−3.