Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Seidl, Wolfgang
and
Breu, Josef
2005.
Single crystal structure refinement of tetramethylammonium-hectorite.
Zeitschrift für Kristallographie - Crystalline Materials,
Vol. 220,
Issue. 2-3,
p.
169.
He, Hongping
Zhou, Qin
Martens, Wayde N.
Kloprogge, Theo J.
Yuan, Peng
Xi, Yunfei
Zhu, Jianxi
and
Frost, Ray L.
2006.
Microstructure of HDTMA+-Modified Montmorillonite and its Influence on Sorption Characteristics.
Clays and Clay Minerals,
Vol. 54,
Issue. 6,
p.
689.
Sanchez-Martin, M.J.
Rodriguez-Cruz, M.S.
Andrades, M.S.
and
Sanchez-Camazano, M.
2006.
Efficiency of different clay minerals modified with a cationic surfactant in the adsorption of pesticides: Influence of clay type and pesticide hydrophobicity.
Applied Clay Science,
Vol. 31,
Issue. 3-4,
p.
216.
Burzo, E.
2007.
Phyllosilicates.
p.
366.
Fuller, Megan
Smith, James A.
and
Burns, Susan E.
2007.
Sorption of nonionic organic solutes from water to tetraalkylammonium bentonites: Mechanistic considerations and application of the Polanyi–Manes potential theory.
Journal of Colloid and Interface Science,
Vol. 313,
Issue. 2,
p.
405.
Zhou, Qin
Frost, Ray L.
He, Hongping
Xi, Yunfei
and
Zbik, Marek
2007.
TEM, XRD, and thermal stability of adsorbed paranitrophenol on DDOAB organoclay.
Journal of Colloid and Interface Science,
Vol. 311,
Issue. 1,
p.
24.
Önal, Müşerref
and
Sarıkaya, Yüksel
2007.
Some physicochemical properties of partition nanophase formed in sorptive organoclays.
Colloids and Surfaces A: Physicochemical and Engineering Aspects,
Vol. 296,
Issue. 1-3,
p.
216.
Liauw, Christopher Mark
Lees, Graham Clayton
Rothon, Roger Norman
Wilkinson, Arthur Norman
and
Limpanapittayatorn, Pipat
2007.
Evaluation of an alternative modification route for layered silicates and synthesis of poly(styrene) layered silicate nanocomposites by in-situ suspension polymerisation.
Composite Interfaces,
Vol. 14,
Issue. 4,
p.
361.
Xu, Liheng
and
Zhu, Lizhong
2007.
Structures of hexamethonium exchanged bentonite and the sorption characteristics for phenol.
Colloids and Surfaces A: Physicochemical and Engineering Aspects,
Vol. 307,
Issue. 1-3,
p.
1.
Önal, Müşerref
and
Sarıkaya, Yüksel
2008.
Some physicochemical properties of methylammonium and ethylenediammonium smectites.
Colloids and Surfaces A: Physicochemical and Engineering Aspects,
Vol. 312,
Issue. 1,
p.
56.
Zhou, Qin
He, Hong Ping
Zhu, Jian Xi
Shen, Wei
Frost, Ray L.
and
Yuan, Peng
2008.
Mechanism of p-nitrophenol adsorption from aqueous solution by HDTMA+-pillared montmorillonite—Implications for water purification.
Journal of Hazardous Materials,
Vol. 154,
Issue. 1-3,
p.
1025.
Ruan, Xiuxiu
Zhu, Lizhong
and
Chen, Baoliang
2008.
Adsorptive Characteristics of the Siloxane Surfaces of Reduced-Charge Bentonites Saturated with Tetramethylammonium Cation.
Environmental Science & Technology,
Vol. 42,
Issue. 21,
p.
7911.
Önal, Müşerref
and
Sarıkaya, Yüksel
2008.
The effect of organic cation content on the interlayer spacing, surface area and porosity of methyltributylammonium-smectite.
Colloids and Surfaces A: Physicochemical and Engineering Aspects,
Vol. 317,
Issue. 1-3,
p.
323.
Ozcan, A. Safa
and
Ozcan, Adnan
2008.
Adsorption of Acid Yellow 99 onto DEDMA-sepiolite from aqueous solutions.
International Journal of Environment and Pollution,
Vol. 34,
Issue. 1/2/3/4,
p.
308.
Zhu, Lizhong
Ruan, Xiuxiu
Chen, Baoliang
and
Zhu, Runliang
2008.
Efficient removal and mechanisms of water soluble aromatic contaminants by a reduced-charge bentonite modified with benzyltrimethylammonium cation.
Chemosphere,
Vol. 70,
Issue. 11,
p.
1987.
Önal, M.
and
Sarıkaya, Y.
2008.
Thermal characterization of methyltributylammonium-smectites.
Journal of Thermal Analysis and Calorimetry,
Vol. 91,
Issue. 3,
p.
835.
Vidal, N.C.
and
Volzone, C.
2009.
Analysis of tetramethylammonium–montmorillonite and retention of toluene from aqueous solution.
Applied Clay Science,
Vol. 45,
Issue. 4,
p.
227.
Seki, Yoko
Okada, Tomohiko
and
Ogawa, Makoto
2009.
Preparation and vapor adsorption properties of quaternary diammonium-montmorillonites.
Microporous and Mesoporous Materials,
Vol. 124,
Issue. 1-3,
p.
30.
Seki, Yoko
and
Ogawa, Makoto
2010.
The Removal of 2-Phenylphenol from Aqueous Solution by Adsorption onto Organoclays.
Bulletin of the Chemical Society of Japan,
Vol. 83,
Issue. 6,
p.
712.
Jović-Jovičić, N.
Milutinović-Nikolić, A.
Banković, P.
Dojčinović, B.
Nedić, B.
Gržetić, I.
and
Jovanović, D.
2010.
Synthesis, Characterization and Adsorptive Properties οf Organobentonites.
Acta Physica Polonica A,
Vol. 117,
Issue. 5,
p.
849.
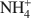 cations defines their packing configurations in the interlayers and the basal spacings of organoclays, and hence strongly influences the sorptive properties of organoclays. A series of organoclays (TAA-SACs) was prepared from a smectite (SAC) fully exchanged with symmetrical tetraalkylammonium (TAA) cations of progressively increasing sizes with the carbon number of single alkyl group from 1 to 6. X-ray diffraction analysis indicated the packing configurations of monolayer, monolayer-to-bilayer transition, bilayer, and bilayer-to-trilayer transition in the interlayers of SAC. Calculations of the interionic distances between TAA ions support such packing configurations. Sorption of benzene by TAA-SACs displayed a high-low-high uptake trend and progressively weaker sorptive interactions as the size of TAA ions increased. Both the siloxane surfaces and TAA ions contributed to the overall sorption, with their relative contributions dependent on the TAA interionic distances and the basal spacings of TAA-SACs. High benzene sorption by small TAA-SAC (tetramethylammonium(TMA)-SAC) was attributed to the strong interactions between the siloxane surfaces and benzene molecules. With large TAAs, high sorption was due to the effective solute partitioning. Compared to benzene sorption, TCE sorption by small TAA-SACs (TMA-SAC and tetraethylammonium(TEA)-SAC) was less effective and displayed an abnormal trend, due largely to the lack of the siloxane surface-TCE interactions and to the stronger hydration of TMA as compared to TEA ions. The results provide strong evidence to support the use of either small or large quaternary NH4+
cations defines their packing configurations in the interlayers and the basal spacings of organoclays, and hence strongly influences the sorptive properties of organoclays. A series of organoclays (TAA-SACs) was prepared from a smectite (SAC) fully exchanged with symmetrical tetraalkylammonium (TAA) cations of progressively increasing sizes with the carbon number of single alkyl group from 1 to 6. X-ray diffraction analysis indicated the packing configurations of monolayer, monolayer-to-bilayer transition, bilayer, and bilayer-to-trilayer transition in the interlayers of SAC. Calculations of the interionic distances between TAA ions support such packing configurations. Sorption of benzene by TAA-SACs displayed a high-low-high uptake trend and progressively weaker sorptive interactions as the size of TAA ions increased. Both the siloxane surfaces and TAA ions contributed to the overall sorption, with their relative contributions dependent on the TAA interionic distances and the basal spacings of TAA-SACs. High benzene sorption by small TAA-SAC (tetramethylammonium(TMA)-SAC) was attributed to the strong interactions between the siloxane surfaces and benzene molecules. With large TAAs, high sorption was due to the effective solute partitioning. Compared to benzene sorption, TCE sorption by small TAA-SACs (TMA-SAC and tetraethylammonium(TEA)-SAC) was less effective and displayed an abnormal trend, due largely to the lack of the siloxane surface-TCE interactions and to the stronger hydration of TMA as compared to TEA ions. The results provide strong evidence to support the use of either small or large quaternary NH4+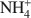 cations in preparation of organoclays as effective sorbents for removing organic contaminants from water.
cations in preparation of organoclays as effective sorbents for removing organic contaminants from water.

