Published online by Cambridge University Press: 01 January 2024
Bentonites are commonly used as chemical containment barriers to minimize liquid flow and contaminant transport. However, chemicals can adversely affect bentonite performance to the extent that modified bentonites have been developed to improve chemical resistance relative to traditional (unmodified) bentonites. The present study focused on the diffusion of potassium chloride (KCl) through a bentonite-polymer composite, or BPC, that was known to behave as a semipermeable membrane. Specifically, the effective diffusion coefficients, D*, for chloride (Cl−) and potassium (K+) were measured and correlated with previously measured membrane efficiency coefficients, ω, for the BPC. The values of D* at steady-state for chloride (Dss,Cl−*\$\end{document}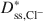 ) and potassium (Dss,K+*\$\end{document}
) and potassium (Dss,K+*\$\end{document}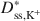 ) decreased as the ω values increased. The decrease in Dss,Cl−*\$\end{document}
) decreased as the ω values increased. The decrease in Dss,Cl−*\$\end{document}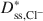 and Dss,K+*\$\end{document}
and Dss,K+*\$\end{document}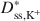 was approximately a linear function of (1 − ω), which is consistent with previous research performed on unmodified Na-bentonite contained within a geosynthetic clay liner (GCL). In contrast to the previous GCL tests, however, Dss,Cl−*\$\end{document}
was approximately a linear function of (1 − ω), which is consistent with previous research performed on unmodified Na-bentonite contained within a geosynthetic clay liner (GCL). In contrast to the previous GCL tests, however, Dss,Cl−*\$\end{document}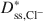 values for the BPC generally were greater than the Dss,K+*\$\end{document}
values for the BPC generally were greater than the Dss,K+*\$\end{document}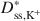 values, and the differences between Dss,Cl−*\$\end{document}
values, and the differences between Dss,Cl−*\$\end{document}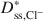 and Dss,K+*\$\end{document}
and Dss,K+*\$\end{document}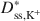 decreased as KCl concentration increased. The apparent discrepancy between Dss,Cl−*\$\end{document}
decreased as KCl concentration increased. The apparent discrepancy between Dss,Cl−*\$\end{document}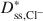 and Dss,K+*\$\end{document}
and Dss,K+*\$\end{document}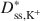 is consistent with excess sodium (Na+) in the BPC prior to testing and the requirement for electroneutrality during testing. Also, despite an apparent linear trend in diffusive mass flux for K+, lack of agreement between the ratio of the diffusive mass flux of K+ relative to that for Cl− as required on the basis of electroneutrality at steady state suggested that steadystate diffusive mass flux for K+ had probably not been achieved due to continual K+-for-Na+ cation exchange. Nonetheless, the excess Na+ and bentonite modification did not affect the fundamental correlation between D* and ω, which requires that D* approaches zero as ω approaches unity (D* → 0 as ω → 1).
is consistent with excess sodium (Na+) in the BPC prior to testing and the requirement for electroneutrality during testing. Also, despite an apparent linear trend in diffusive mass flux for K+, lack of agreement between the ratio of the diffusive mass flux of K+ relative to that for Cl− as required on the basis of electroneutrality at steady state suggested that steadystate diffusive mass flux for K+ had probably not been achieved due to continual K+-for-Na+ cation exchange. Nonetheless, the excess Na+ and bentonite modification did not affect the fundamental correlation between D* and ω, which requires that D* approaches zero as ω approaches unity (D* → 0 as ω → 1).