Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Kogure, Toshihiro
Hybler, Jiří
and
Yoshida, Hideto
2002.
Coexistence of Two Polytypic Groups in Cronstedtite From Lostwithiel, England.
Clays and Clay Minerals,
Vol. 50,
Issue. 4,
p.
504.
Ďurovič, Slavomil
Hybler, Jiří
and
Kogure, Toshihiro
2004.
Parallel intergrowths in cronstedtite-1T: Implications for structure refinement.
Clays and Clay Minerals,
Vol. 52,
Issue. 5,
p.
613.
Ďurovič, Slavomil
and
Hybler, Jiři
2006.
OD structures in crystallography — basic concepts and suggestions for practice.
Zeitschrift für Kristallographie - Crystalline Materials,
Vol. 221,
Issue. 1,
p.
63.
Burzo, E.
2009.
Phyllosilicates.
Vol. 27I5b,
Issue. ,
p.
258.
Wahle, Michael W.
Bujnowski, Thomas J.
Guggenheim, Stephen
and
Kogure, Toshihiro
2010.
Guidottiite, the Mn-Analogue of Cronstedtite: A New Serpentine-Group Mineral from South Africa.
Clays and Clay Minerals,
Vol. 58,
Issue. 3,
p.
364.
Kogure, T.
and
Okunishi, E.
2010.
Cs-corrected HAADF-STEM imaging of silicate minerals.
Journal of Electron Microscopy,
Vol. 59,
Issue. 4,
p.
263.
Brigatti, Maria Franca
Malferrari, Daniele
Laurora, Angela
and
Elmi, Chiara
2011.
Layered Mineral Structures and their Application in Advanced
Technologies.
p.
1.
Guggenheim, Stephen
2011.
Layered Mineral Structures and their Application in Advanced
Technologies.
p.
73.
Elmaleh, Agnès
Tarantino, Serena Chiara
Zema, Michele
Devouard, Bertrand
and
Fialin, Michel
2012.
The low‐temperature magnetic signature of Fe‐rich serpentine in CM2 chondrites: Comparison with terrestrial cronstedtite and evolution with the degree of alteration.
Geochemistry, Geophysics, Geosystems,
Vol. 13,
Issue. 5,
Pignatelli, I.
Mugnaioli, E.
Hybler, J.
Mosser-Ruck, R.
Cathelineau, M.
and
Michau, N.
2013.
A Multi-Technique Characterization of Cronstedtite Synthesized by Iron-Clay Interaction in a Step-By-Step Cooling Procedure.
Clays and Clay Minerals,
Vol. 61,
Issue. 4,
p.
277.
Hybler, Jiří
2014.
Refinement of cronstedtite-1M.
Acta Crystallographica Section B Structural Science, Crystal Engineering and Materials,
Vol. 70,
Issue. 6,
p.
963.
Elmaleh, Agnès
Bourdelle, Franck
Caste, Florent
Benzerara, Karim
Leroux, Hugues
and
Devouard, Bertrand
2015.
Formation and transformations of Fe-rich serpentines by asteroidal aqueous alteration processes: A nanoscale study of the Murray chondrite.
Geochimica et Cosmochimica Acta,
Vol. 158,
Issue. ,
p.
162.
Sutton, S.
Alexander, C.M.O'D.
Bryant, A.
Lanzirotti, A.
Newville, M.
and
Cloutis, E.A.
2017.
The bulk valence state of Fe and the origin of water in chondrites.
Geochimica et Cosmochimica Acta,
Vol. 211,
Issue. ,
p.
115.
Pignatelli, Isabella
Marrocchi, Yves
Mugnaioli, Enrico
Bourdelle, Franck
and
Gounelle, Matthieu
2017.
Mineralogical, crystallographic and redox features of the earliest stages of fluid alteration in CM chondrites.
Geochimica et Cosmochimica Acta,
Vol. 209,
Issue. ,
p.
106.
Hybler, Jiří
Klementová, Mariana
Jarošová, Markéta
Pignatelli, Isabella
Mosser-Ruck, Régine
and
Ďurovič, Slavomil
2018.
Polytype Identification in Trioctahedral 1:1 Layer Silicates Using Electron Diffraction with Application to a Chronstedtite That was Synthesized Using Metallic Iron-Clay Interactions.
Clays and Clay Minerals,
Vol. 66,
Issue. 4,
p.
379.
Hybler, Jiří
Dolníček, Zdeněk
Sejkora, Jiří
and
Števko, Martin
2020.
Polytypism of Cronstedtite From Nagybörzsöny, Hungary.
Clays and Clay Minerals,
Vol. 68,
Issue. 6,
p.
632.
Pignatelli, Isabella
Mosser-Ruck, Régine
Mugnaioli, Enrico
Sterpenich, Jérôme
and
Gemmi, Mauro
2020.
The Effect of the Starting Mineralogical Mixture on the Nature of Fe-Serpentines Obtained during Hydrothermal Synthesis AT 90°C.
Clays and Clay Minerals,
Vol. 68,
Issue. 4,
p.
394.
Hybler, Jiří
Dolníček, Zdeněk
Sejkora, Jiří
and
Števko, Martin
2021.
Polytypism of Cronstedtite from Ouedi Beht, El Hammam, Morocco.
Clays and Clay Minerals,
Vol. 69,
Issue. 6,
p.
702.
Yakich, T. Yu.
Zhimuleva, E. S.
Rudmin, M. A.
Ruban, A. S.
Maximov, P. N.
and
Shaldybin, M. V.
2023.
The first identification of cronstedtite in Cu–Ni–PGE ores of the Talnakh intrusion.
Scientific Reports,
Vol. 13,
Issue. 1,
Pignatelli, Isabella
Mugnaioli, Enrico
Mosser-Ruck, Régine
Abdelmoula, Mustapha
and
Sterpenich, Jérôme
2024.
Cronstedtite: H2 generation and new constraints on its formation conditions.
Applied Clay Science,
Vol. 262,
Issue. ,
p.
107627.
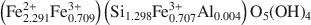 and the Příbram sample has the composition (Fe2.2692+Fe0.7313+)(Si1.271Fe0.7243+Al0.005)O5(OH)4
and the Příbram sample has the composition (Fe2.2692+Fe0.7313+)(Si1.271Fe0.7243+Al0.005)O5(OH)4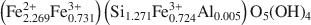 . The results of refinements are as follows: a = 5.500(1), c = 14.163(2) Å, V = 371.08(8) Å3, R = 3.83%, from 381 independent reflections, and a = 5.4927(1), c = 14.1481(2) Å, V = 369.70(4) Å3, R = 4.77%, from 1088 independent reflections for the Wheal Maudlin and Příbram samples, respectively. The best Fovs.Fc agreement was achieved when the structure was interpreted as merohedral twin; several possible twinning laws are discussed. The cronstedtite layer consists of one tetrahedral sheet and one octahedral sheet. There is one octahedral (M1) position, occupied by Fe only, and two tetrahedral (T1, T2) positions in the structure. Refinement of occupancy of tetrahedral sites led to values Si:Fe = 0.45:0.55(1) (Wheal Maudlin) and 0.432:0.568(8) (Příbram) in T1, and Si: Fe = 0.99:0.01(1) (Wheal Maudlin) and 0.888:0.112(7) (Příbram) in 72. Whereas the size of T1 is reasonable (average dT1-O = 1.693 Å (Wheal Maudlin), 1.691 Å (Příbram)), T2 is unusually large: (dT2-O= 1.740 Å (Wheal Maudlin), 1.737 Å (Příbram)) with respect to the small or almost zero Fe content. As an explanation, an alternative structure model comprising a certain amount of vacancies in T2 is presented. The tetrahedral rotation angle α is highly positive (+12.1° and +12.5° for the Wheal Maudlin and Příbram samples, respectively), and the layer belongs to the Franzini type A. Distortion parameters of octahedra and tetrahedra are given for both samples. One hydrogen atom engaged in the hydrogen bond was located in the Wheal Maudlin sample.
. The results of refinements are as follows: a = 5.500(1), c = 14.163(2) Å, V = 371.08(8) Å3, R = 3.83%, from 381 independent reflections, and a = 5.4927(1), c = 14.1481(2) Å, V = 369.70(4) Å3, R = 4.77%, from 1088 independent reflections for the Wheal Maudlin and Příbram samples, respectively. The best Fovs.Fc agreement was achieved when the structure was interpreted as merohedral twin; several possible twinning laws are discussed. The cronstedtite layer consists of one tetrahedral sheet and one octahedral sheet. There is one octahedral (M1) position, occupied by Fe only, and two tetrahedral (T1, T2) positions in the structure. Refinement of occupancy of tetrahedral sites led to values Si:Fe = 0.45:0.55(1) (Wheal Maudlin) and 0.432:0.568(8) (Příbram) in T1, and Si: Fe = 0.99:0.01(1) (Wheal Maudlin) and 0.888:0.112(7) (Příbram) in 72. Whereas the size of T1 is reasonable (average dT1-O = 1.693 Å (Wheal Maudlin), 1.691 Å (Příbram)), T2 is unusually large: (dT2-O= 1.740 Å (Wheal Maudlin), 1.737 Å (Příbram)) with respect to the small or almost zero Fe content. As an explanation, an alternative structure model comprising a certain amount of vacancies in T2 is presented. The tetrahedral rotation angle α is highly positive (+12.1° and +12.5° for the Wheal Maudlin and Příbram samples, respectively), and the layer belongs to the Franzini type A. Distortion parameters of octahedra and tetrahedra are given for both samples. One hydrogen atom engaged in the hydrogen bond was located in the Wheal Maudlin sample.

