Introduction
Heavy-metal pollution of surface and underground water sources has threatened ecological environments and human health (Chen et al. Reference Chen, Zeng, Xu, Shen, Hu, Zhu, Du and Sun2018; Luo et al. Reference Luo, Luo, Crittenden, Qu, Bai, Peng and Li2015, Reference Luo, Sun, Ritt, Liu, Pei, Crittenden and Elimelech2019; Wang et al. Reference Wang, Liu, Fu, Yi, Wang, Zhao, Wang and Zheng2019). As a typical, toxic, heavy-metal ion, hexavalent chromium (Cr(VI)), derived mainly from chromate-related industrial processes such as wood treatment, leather tanning, and textile industries, has received significant attention in the literature (Nanda et al. Reference Nanda, Pradhan and Parida2017; Velegraki et al. Reference Velegraki, Miao, Drivas, Liu, Kennou and Armatas2018; Yuan et al. Reference Yuan, Yue, Qiu, Liu and Li2019). Semiconductor-mediated photocatalytic reduction is considered to be a clean way to remove toxic Cr(VI) from wastewater (Jing et al. Reference Jing, Liang, Xiong, Chen, Zhang, Li and Wu2017; Zhong et al. Reference Zhong, Qiu, Chen, Li, Xu, Li, He and Lu2016). As a silver-based photocatalyst, Ag3PO4 has aroused great attention due to the large quantum yield and the visible-light response (Chen et al. Reference Chen, Zeng, Yi, Xiao, Xu, Shen and Feng2019a; Shao et al. Reference Shao, Liu, Liu, Zeng, Liang, Liang, Cheng, Zhang, Liu and Gong2019). Some problems remain, however, during the photocatalytic process: (1) the high recombination rate of the photogenerated electron holes (Zhou et al. Reference Zhou, Zhang, Chen and Deng2017); (2) the serious photocorrosion phenomenon (Ag3PO4 → Ag0) (Xu et al. Reference Xu, Wang, Song, Zhu, Xu, Yan, Song and Li2014); and (3) the relatively large particle size of Ag3PO4 (Abroshan et al. Reference Abroshan, Farhadi and Zabardasti2018). Many studies involving the combination of Ag3PO4 with other semiconductor materials to construct heterojunction composites have attempted to solve these problems (Dhanabal et al. Reference Dhanabal, Velmathi and Chandra2018; Kumar et al. Reference Kumar, Surendar, Baruah and Shanker2013; Liu et al. Reference Liu, Xu, Ni, Wang, You and Guo2019a; Tian et al. Reference Tian, Yang, Cui, Liu and Tang2019; Wan et al. Reference Wan, Du, Liu, Hu, Fan and Hu2017). Although the photocatalytic activity of these hybrid composites has been improved to some degree above that of pure Ag3PO4, a more stable and more efficient Ag3PO4-based photocatalyst is still needed.
Layered double hydroxides (LDHs), consisting of positively charged brucite-like layers and negatively charged interlayer anions, have attracted much attention in photocatalysis and photoelectrocatalysis (Wu et al. Reference Wu, Wang, Sun, Xiao, Tu, Yuan, Zeng, Li and Chew2018; Zhao et al. Reference Zhao, Chen, Bian, Zhou, Waterhouse, Wu, Tung, Smith, O'Hare and Zhang2015, Reference Zhao, Jia, Waterhouse, Wu, Tung, O'Hare and Zhang2016). In previous work, ZnAl LDH was shown to be an effective catalyst support for the photocatalyst due to its 2D layered structure, large surface area, and abundant hydroxyl groups (Chen et al. Reference Chen, Zeng, Yi, Xiao, Zhu, Cao, Shen and Peng2019b). The net trap confinement effects of LDH can inhibit effectively the aggregation of particles. Because of its band gap of 2.1 eV, CoAl LDH is considered to offer promise as a photocatalyst; it is also low-cost, environmentally friendly, and has appropriate oxidation–reduction potential (Kumar et al. Reference Kumar, Isaacs, Trofimovaite, Durndell, Parlett, Douthwaite, Coulson, Cockett, Wilson and Lee2017; Li et al. Reference Li, Li, Xu, Yang, Ng, Song and Zuo2017; Wang et al. Reference Wang, Zhang, Su, Shao, Zeng and Wang2018). The relatively poor electrical conductivity and high electrical resistance of CoAl LDH restrict severely its photocatalytic application, however (Fang et al. Reference Fang, Li, Li, Zhang, Shou, Liu, Zhang and Cheng2012; Peng et al. Reference Peng, Li and Song2017a).
To solve these problems, some studies focused on incorporation in CoAl LDH of conductive carbon materials, e.g. carbon nanotubes (Zhang et al. Reference Zhang, Li, Sun, Liu, Yang and Zhao2016a), graphitic carbon nitride (Jo and Tonda Reference Jo and Tonda2019), and fluorinated graphene (Peng et al. Reference Peng, Li and Song2017a), which resulted in significant improvement in the electrical conductivity and electrical resistance. The introduction of conductive carbon material might, therefore, be an effective strategy for enhancing the photocatalytic performances of heterojunction photocatalysts. As a metal-free conjugated polymer semiconductor, graphitic carbon nitride (g-C3N4) has become a focus of attention due to its appropriate electronic band structure, low cost, and easy preparation (Li et al. Reference Li, Huang, Zhu, Luo, Yan, Dai, Guo and Yang2019; Zheng et al. Reference Zheng, Lin, Wang and Wang2015). Moreover, g-C3N4 with its unique delocalized π-conjugated structure and good conductivity can provide a larger interface contact area and promote photogenerated charge separation (Jo and Tonda Reference Jo and Tonda2019; Shakeel et al. Reference Shakeel, Arif, Yasin, Li and Khan2019).
Based on the discussion above, the objectives of the present study were to design a multiple heterojunction system of g-C3N4/LDH/Ag3PO4 (CNLDHAP) composite which could be constructed via a two-step hydrothermal pathway, to evaluate its photocatalytic activity toward Cr(VI) reduction under visible light irradiation, and to confirm the stability and reusability of the CNLDHAP composite upon reuse or recycling. A final objective was to propose a probable mechanism by which the photocatalysis occurs.
EXPERIMENTS
Materials
Co(NO3)2·6H2O, Al(NO3)3·9H2O, Na2HPO4, AgNO3, NaOH, HCl, and urea were purchased from Sinopharm Chemical Reagent Co., Ltd. China; K2Cr2O7 was purchased from Shanghai Macklin Biochemical Technology Co., Ltd. China. All the chemical reagents were of analytical grade and used without further purification.
Synthesis of g-C 3 N 4
Pure g-C3N4 was prepared by the thermal polymerization of urea. 15 g of urea was placed in a crucible with a cover, and heated at 550°C for 3 h with a heating rate of 5°C min–1. After cooling naturally to room temperature, the yellow product was ground to powder and the g-C3N4 obtained.
Synthesis of g-C 3 N 4 /LDH/Ag 3 PO 4 Composites
The preparation process of g-C3N4/LDH/Ag3PO4 composite was as illustrated in Fig. 1. Briefly, g-C3N4 (80 mg) was mixed in 80 mL of deionized water and treated ultrasonically for 60 min. Co(NO3)2·6H2O (1.833 g), Al(NO3)3·9H2O (0.788 g), and urea (3.405 g) were added to the solutions above and stirred for 120 min, and then heated at 110°C for 24 h. The sample obtained was denoted as g-C3N4/LDH. Subsequently, 0.8 g of g-C3N4/LDH was dispersed in 50 mL of deionized water and an appropriate amount of AgNO3 (0.380, 0.630, 0.882, or 1.13 g) was dissolved in the above suspensions and stirred for 120 min. After that, 20 mL of a certain concentration of Na2HPO4 (0.037, 0.062, 0.087, or 0.111 M) was added dropwise to the mixture solution and stirred for 12 h at room temperature. Finally, the resulting suspension was centrifuged at 4192.5 × g and washed thoroughly with deionized water, and dried in vacuum at 60°C overnight. According to the calculated weight percent of Ag+ in the g-C3N4/LDH, a series of g-C3N4/LDH/Ag3PO4 composites with various weight percentages of Ag+ were denoted as CNLDHAP30, CNLDHAP50, CNLDHAP70, and CNLDHAP90, respectively. For comparison, the Ag3PO4 and LDH were also synthesized following a similar procedure.
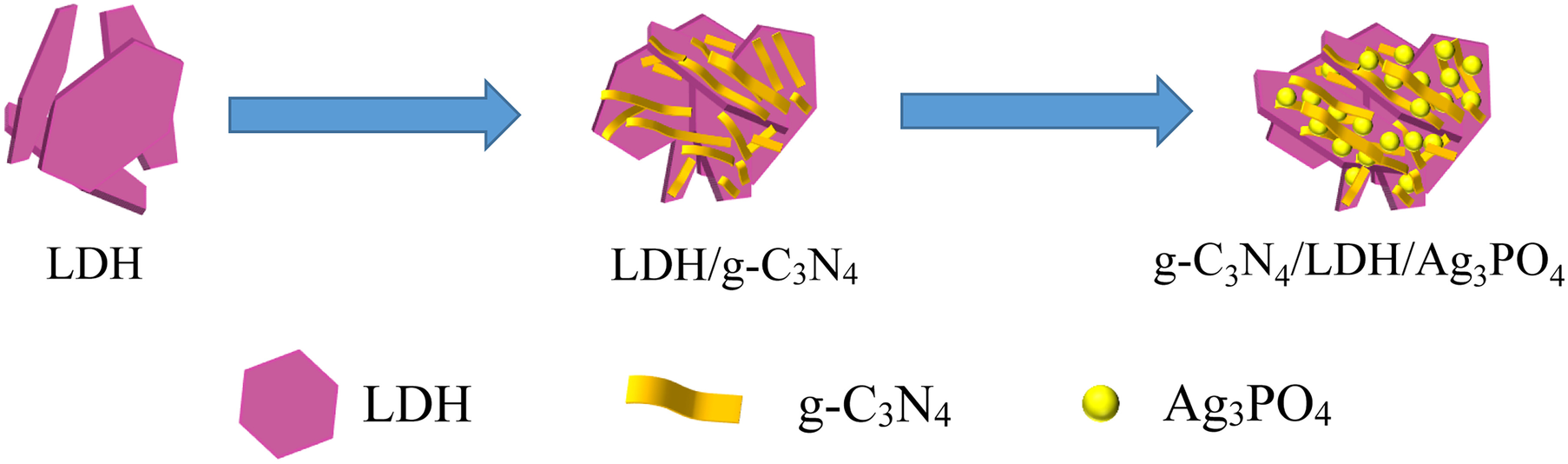
Fig. 1 Schematic diagram illustrating the fabrication process for the g-C3N4/LDH/Ag3PO4 composite
Characterization
Powder X-ray diffraction (XRD) measurements were performed using a Rigaku D/max 2550PC (Tokyo, Japan) instrument with CuKα irradiation (λ = 1.5405 Å). The scan step was 0.02°2θ with a filament current of 30 mA and a voltage of 40 kV. Scanning electron microscopy (JEOL JSM-6700F, Tokyo, Japan) and transmission electron microscopy (FEI Talos F200S, Waltham, Massachusetts, USA) were used to examine the morphologies and microstructures of the samples. X-ray photoelectron spectroscopy (XPS) analyses were carried out using a Thermo Fisher Scientific K-Alpha spectrometer (Waltham, Massachusetts, USA). The C 1s peak from the adventitious carbon-based contaminant with a binding energy of 284.8 eV was used as the reference for calibration. UV-Visible diffuse reflectance spectra (UV-Vis DRS) were measured by using a UV-Visible spectrophotometer (Shimadzu UV-2550, Tokyo, Japan). Photoluminescence (PL) spectra were recorded using a fluorescence spectrophotometer (F-4600, Tokyo, Japan) at room temperature. The scanning speed was 1200 nm/min and the width of the emission slit was 5.0 nm. Mott-Schottky plots were created using an electrochemical workstation (CHI660E, Shanghai Chenhua Instrument Co. Ltd., China) and the standard three-electrode system was immersed in 0.5 mol L–1 Na2SO4 solution at room temperature. During the measurements, a saturated calomel electrode (SCE) served as the reference electrode and Pt foil as the counter electrode. The working electrodes were prepared by coating the indium-doped tin oxide (ITO) conductive glass with g-C3N4, Ag3PO4, and LDH ethanol solution.
Photocatalytic Experiments
Photocatalytic activities of the as-synthesized photocatalysts were evaluated by examining the photoreduction efficiency of Cr(VI). A 300 W Xe lamp equipped with a 420 nm cut-off filter was used as the visible light source. In the photocatalytic experiments, 100 mg of photocatalyst was dispersed in 100 mL of 50 mg⋅L–1 Cr(VI) aqueous solution. Prior to irradiation, the mixed solution was stirred vigorously in the dark for 30 min to establish an adsorption–desorption equilibrium between the photocatalyst and Cr(VI). At regular time intervals (15 min), 2 mL of the reaction solution was collected and centrifuged at 16,770 × g. Subsequently, the Cr(VI) content was determined by the 1,5-diphenylcarbazide method using a UV-Vis spectrophotometer (752 N spectrophotometer, Shanghai Spectrum Instruments Co., Ltd., China) at 540 nm (Chen et al. Reference Chen, Zeng, Xu, Shen, Hu, Zhu, Du and Sun2018).
RESULTS AND DISCUSSION
Structural Characterization
The phase structures of the g-C3N4, Ag3PO4, LDH, g-C3N4/LDH, CNLDHAP30, CNLDHAP50, CNLDHAP70, and CNLDHAP90 composites were investigated by XRD (Fig. 2a). The g-C3N4 exhibited diffraction peaks at ~13.1 and 27.3°2θ corresponding to the (100) and (002) planes of g-C3N4 (Sun et al. Reference Sun, Hu, Zheng, Zhang and Sheng2019). For the Ag3PO4, the diffraction peaks located at 20.9, 29.7, 33.3, 36.6, 47.8, 52.7, 55.2, 57.2, 61.6, and 71.8°2θ were attributed to the (110), (200), (210), (211), (310), (222), (320), (321), (400), and (421) planes of the body-centered cubic structure of Ag3PO4 (Chen et al. Reference Chen, Zeng, Yi, Xiao, Xu, Shen and Feng2019a). As for LDH, the main peaks at 11.5, 23.2, 34.1, 38.5, 60.1, and 61.2°2θ were well indexed to the (003), (006), (012), (015), (110), and (113) planes of CoAl layered double hydroxides (Jo and Tonda Reference Jo and Tonda2019). For the CNLDHAP composites, all of the characteristic peaks of LDH and Ag3PO4 were observed clearly, indicating that the formation of a ternary composite had no effect on their crystalline structure. No obvious characteristic diffraction peaks of g-C3N4 were detected, so it is either absent or less than the XRD detection limit.
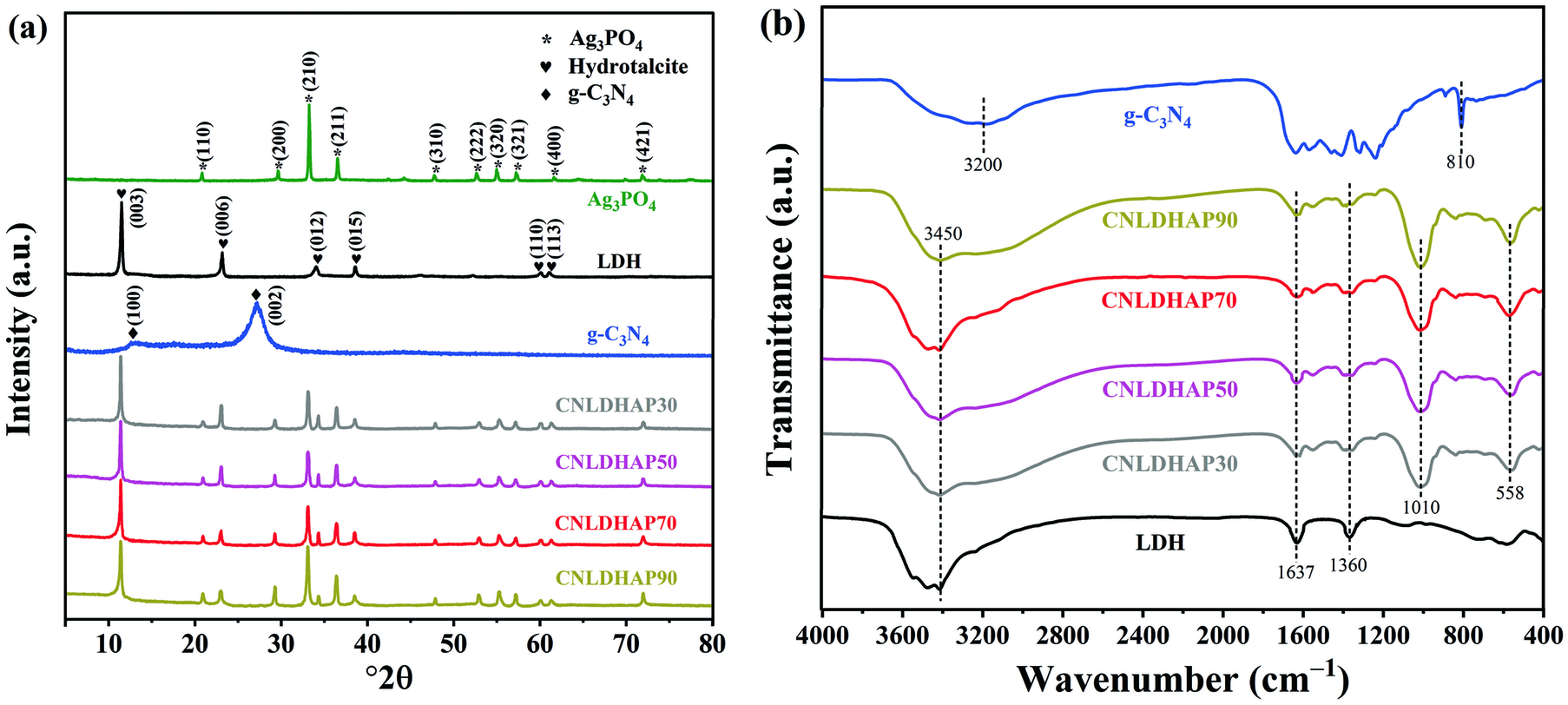
Fig. 2 a XRD patterns of the Ag3PO4, g-C3N4, LDH, and CNLDHAP composites, b FTIR spectra of the g-C3N4, LDH, and CNLDHAP composites
The FTIR spectra were collected to identify bonding information in the samples (Fig. 2b). The peak at ~3200 cm−1 was ascribed to a N − H stretching vibration from the uncondensed amino groups on g-C3N4 (Tonda et al. Reference Tonda, Kumar, Bhardwaj, Yadav and Ogale2018). The peaks in the region 1100 to 1650 cm−1 were assigned to the typical stretching vibration of C − N and C = N heterocycles, and the peak at ~810 cm−1 arose from the triazine rings of g-C3N4 (Wang et al. Reference Wang, Li, Wang, Zhang, Lia and Gong2014). The broad absorption band at ~3450 cm−1 was assigned to the stretching vibrations of surface hydroxyl groups on LDH (Chen et al. Reference Chen, Zeng, Xu, Liu, Duan and Han2017), while the peaks at ~1360 and ~1637 cm−1 were attributed to the bending vibration of CO3 2– and interlayer water molecules of LDH, respectively (Peng et al. Reference Peng, Li and Song2017a; Yang et al. Reference Yang, Li, Liu, Ng, Liu, Sun and Yang2019). The peaks below ~800 cm−1 were ascribed to the stretching and bending vibration of a metal–oxygen bond (Co − O and Al − O) (Peng et al. Reference Peng, Li, Liu and Song2017b). As for the CNLDHAP composites, the peaks at ~558 and ~ 1010 cm−1 were assigned to the asymmetric stretching vibration of P − O in PO4 3−.
The morphological features of g-C3N4, Ag3PO4, LDH, g-C3N4/LDH, and CNLDHAP70 were examined by SEM. The Ag3PO4 exhibited a regular spherical shape with smooth surface, while the g-C3N4 displayed an irregular shape with a corrugated structure (Fig. 3a, b). The LDH (Fig. 3c) showed a thin hexagonal shape with an average lateral size between ~1.5 and 2.0 μm, which conformed to the typical structural characteristic of hydrotalcites. The g-C3N4/LDH (Fig. 3d) showed an open hierarchical structure with the thin hexagonal LDH sheets embedded randomly in the wrinkled g-C3N4. The CNLDHAP70 composite maintained the morphological feature of g-C3N4/LDH and the Ag3PO4 nanoparticles were kept in a well-dispersed state in the composites (Fig. 3e). In addition, the CNLDHAP70 composite was also characterized by TEM (Fig. 3f) and the results revealed that the g-C3N4 crystals overlapped and adhered tightly to the surface of LDH. The close contact among g-C3N4, LDH, and Ag3PO4 indicated a well-formed heterostructure interface between them, which could benefit the separation and transfer of the photogenerated carriers.
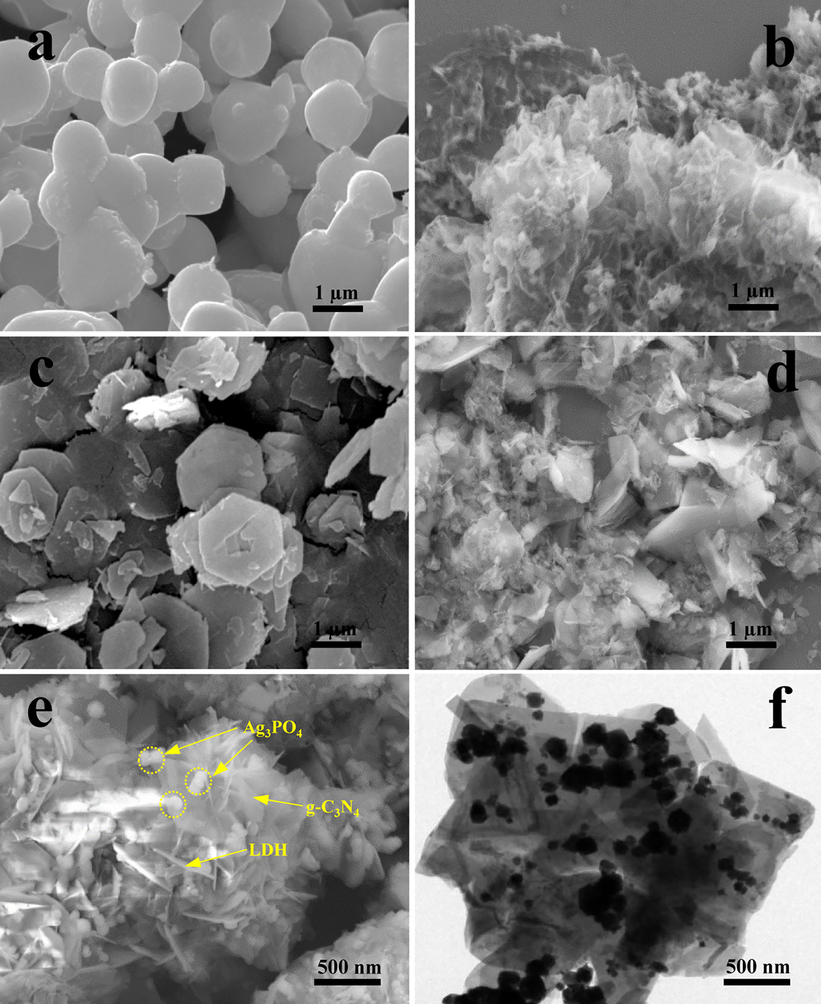
Fig. 3 SEM images of a Ag3PO4, b g-C3N4, c LDH, d g-C3N4/LDH, and e CNLDHAP70; f TEM image of CNLDHAP70
The chemical composition and chemical states of the CNLDHAP70 composite were analyzed by XPS (Fig. 4). The C 1s spectrum could be divided into four different peaks at 284.4, 286.5, 288.2, and 285.5 eV (Fig. 4a), which were ascribed to the C = C, C = O, N = C–N, and C–O bonds, respectively (Shakeel et al. Reference Shakeel, Arif, Yasin, Li and Khan2019; Zhang et al. Reference Zhang, Zhang, Jiang, Liu, Xu, Huo, Zhang and Hu2016b). The two peaks of Ag 3d5/2 and Ag 3d3/2 at 368.4 and 374.4 eV in the Ag 3d spectrum were attributed to Ag+ ions in Ag3PO4 (Fig. 4b), indicating that the silver existed in the form of Ag+ in the CNLDHAP70 (Chen et al. Reference Chen, Zeng, Yi, Xiao, Xu, Shen and Feng2019a). Meanwhile, the peak at 132.8 eV in the P 2p spectrum (Fig. 4c) corresponded to P5+ ions in Ag3PO4 (Tan et al. Reference Tan, Chen, Wu, Shang, Liu, Pan and Xiong2017). For the O 1s spectrum (Fig. 4d), the peaks at 530.4, 531.6, and 532.6 eV were ascribed to the lattice oxygen (O2–), surface –OH groups, and surface-adsorbed water, respectively (Fettkenhauer et al. Reference Fettkenhauer, Clavel, Kailasam, Antonietti and Dontsova2015). The Co 2p spectrum was separated into four peaks according to the Gaussian fitting method (Fig. 4e). The peaks of 780.5 and 796.7 eV were assigned to Co 2p3/2 and Co 2p1/2; the other two peaks at 785.5 and 802.1 eV belonged to the satellite peaks of Co(II), which demonstrated a high-spin Co2+ state in CNLDHAP70 (Ma et al. Reference Ma, Liang, Takada and Sasaki2010). The peak at 73.8 eV in the Al 2p spectrum (Fig. 4f) corresponded well with the Al3+ in the brucite-like layers of LDH (Das et al. Reference Das, Patnaik and Parida2019).
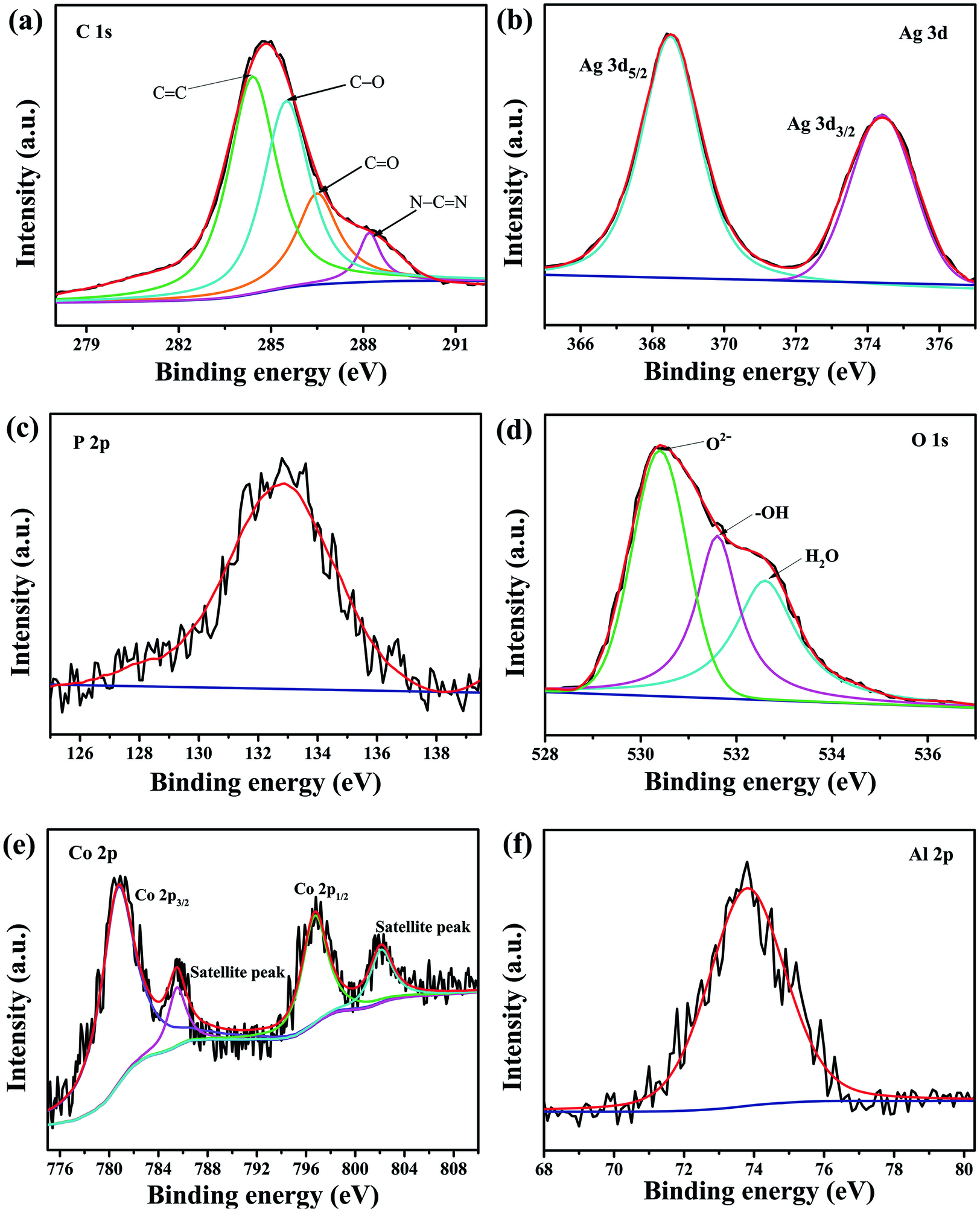
Fig. 4 XPS spectra of the CNLDHAP70 composite: a C 1s, b Ag 3d, c P 3p, d O 1s, e Co 2p, and f Al 2p
The optical absorption properties of Ag3PO4, g-C3N4, LDH, g-C3N4/LDH, and CNLDHAP composites were studied by UV-Vis DRS. The Ag3PO4 and g-C3N4 displayed a broad absorption in the visible region with absorption edges at ~530 and 460 nm, respectively (Fig. 5a). The LDH showed two distinct absorption bands in the whole spectrum: the strong absorption band below 300 nm resulted from ligand-to-metal charge transfer, and the absorption band at ~500 nm corresponded to d–d transitions of octahedral Co2+ within the brucite-like layers of CoAl-layered double hydrotalcites (Wang et al. Reference Wang, Zhang, Su, Shao, Zeng and Wang2018). Compared with the LDH, the g-C3N4/LDH exhibited an improved light absorption in the region between 200 and 450 nm. In particular, the absorption edges of all the CNLDHAP ternary composites demonstrated a remarkable red-shift compared to LDH, and the absorption intensity increased with the amount of Ag3PO4. The results suggested that CNLDHAP ternary composites could be promising as a visible light active photocatalyst. In addition, the band-gap energy (E g) was obtained according to the Kubelka–Munk formula (Wang et al. Reference Wang, Zhang, Su, Shao, Zeng and Wang2018) (Fig. 5b). The E g of g-C3N4, LDH, and Ag3PO4 were 2.65, 2.10, and 2.43 eV, respectively.
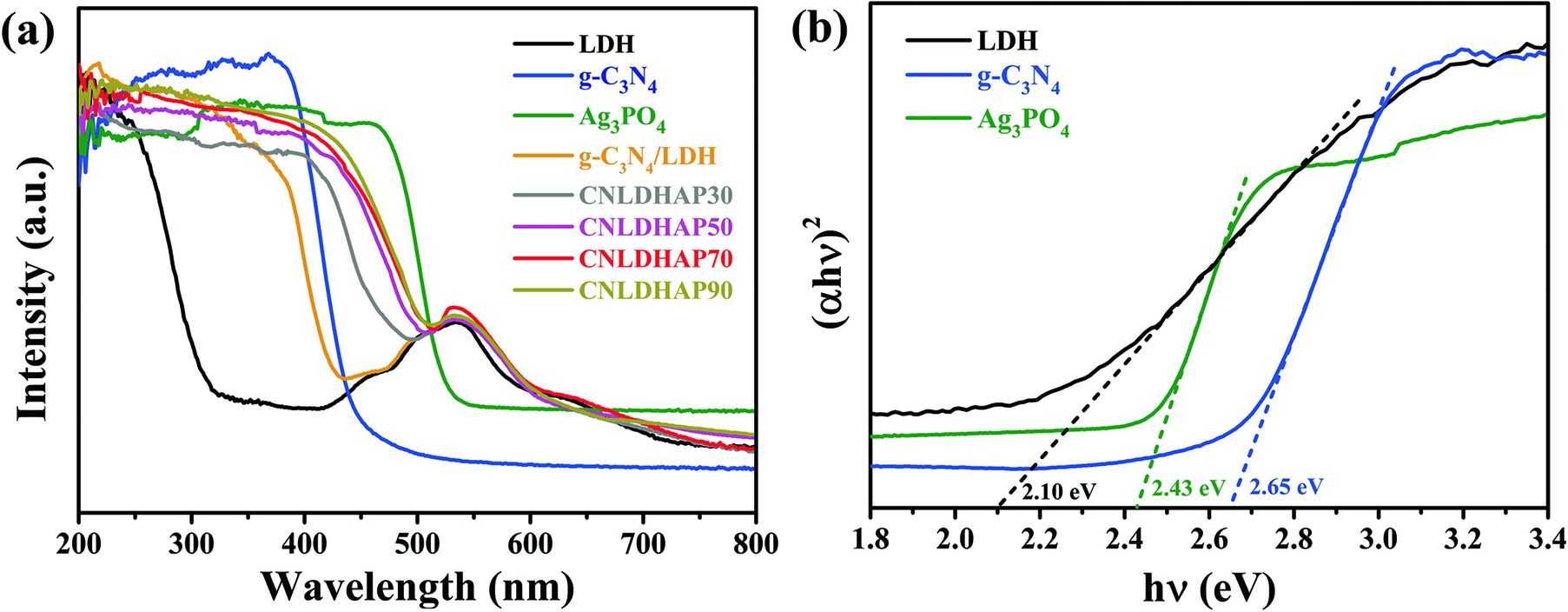
Fig. 5 a UV-vis DRS spectra of the Ag3PO4, g-C3N4, g-C3N4/LDH, and CNLDHAP composites; b Tauc plots of the Ag3PO4, g-C3N4, and LDH
The charge separation efficiency of the products were studied using PL spectra (Fig. 6). The LDH showed two obvious emission peaks centered at ~400 and 448 nm, while the g-C3N4/LDH showed a weaker PL intensity, indicating a more efficient charge transfer and separation in the g-C3N4/LDH. Furthermore, the peak intensity of the CNLDHAP composites decreased further compared with both the LDH and g-C3N4/LDH. In particular, the PL intensity of the ternary composite decreased at first and then increased with increasing amount of Ag3PO4; the CNLDHAP70 showed the lowest PL intensity. This indicated that the moderate Ag3PO4 loading was favorable for charge separation efficiency, but the excessive dosage could become the recombination centers for the photogenerated charge carriers and be unfavorable for the reduction of Cr(VI).
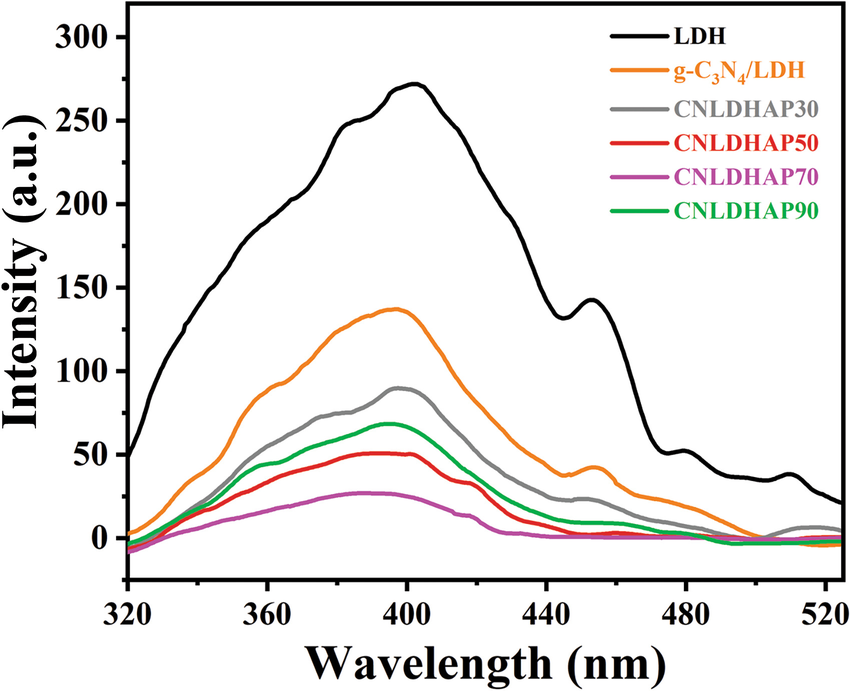
Fig. 6 PL spectra of the LDH, g-C3N4, g-C3N4/LDH, and CNLDHAP composites
Photocatalytic Behaviors on Cr(VI) Reduction
The photocatalytic activity of composite samples was evaluated by reducing Cr(VI) at pH 3 under visible light irradiation. A negligible change of Cr(VI) concentration in the absence of photocatalyst indicated that Cr(VI) aqueous solution was stable under visible light irradiation (Fig. 7a). The Ag3PO4, g-C3N4, and LDH possessed poor photocatalytic activities, and their reduction ratios for Cr(VI) were only 18.1, 23.0, and 33.5% after 120 min of irradiation. Furthermore, the Cr(VI) removal ratio of g-C3N4/LDH reached 56.9%, which indicated that coupling g-C3N4 and LDH was an effective way to improve the photocatalytic reduction activity for Cr(VI). Compared with g-C3N4/LDH, all of the CNLDHAP composites showed much greater photocatalytic-reduction efficiency. In particular, CNLDHAP70 exhibited the greatest photocatalytic activity, and 96.0% of Cr(VI) was reduced within 120 min of visible-light irradiation.
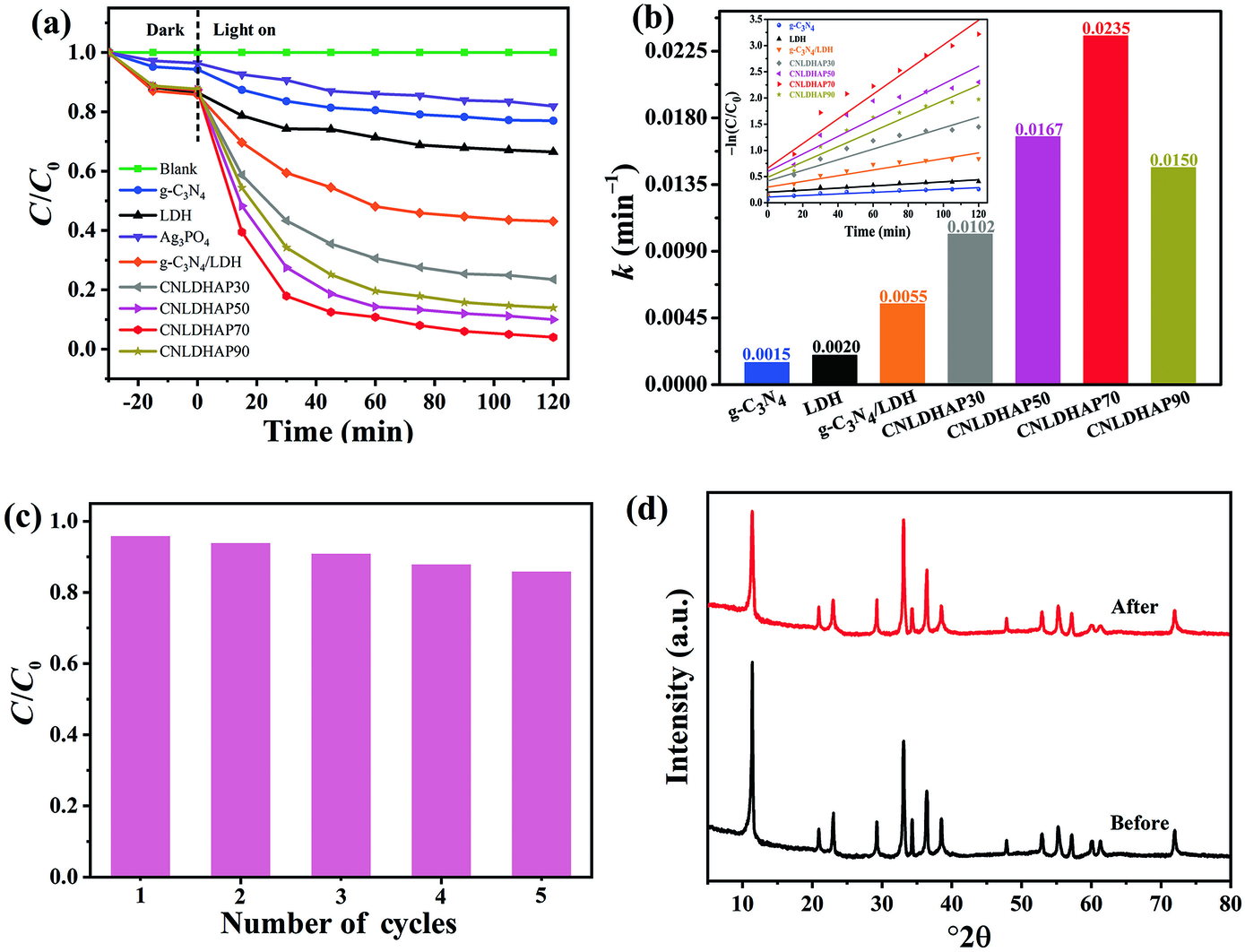
Fig. 7 a Photocatalytic reduction efficiency, b apparent reaction rate constants, pseudo-first order kinetics (inset in b), c reusability for Cr(VI) reduction under visible light irradiation, d XRD patterns of the CNLDHAP70 before and after photocatalytic reaction
The pseudo-first order model (–ln(C/C 0) = kt) was employed to investigate the photocatalytic reduction process (Liu et al. Reference Liu, Zhu, Gao, Hojamberdiev, Zhu, Wei, Guo and Liu2017). The results revealed that CNLDHAP70 had the largest value of k of 0.0235 min–1 (Fig. 7b), which was almost 15.67 and 11.75 times greater than that of g-C3N4 (0.0015 min–1) and LDH (0.0020 min–1), respectively. The CNLDHAP70 was selected for the recycling experiments and the results showed that CNLDHAP70 maintained a significant Cr(VI) reduction activity (86.0%) after five repeats of the experiment. The slight declines in Cr(VI) reduction efficiency were attributed to the active site coverage and to photocatalyst loss during the recycling process. The XRD patterns of CNLDHAP70 before and after Cr(VI) reduction were collected and the results revealed that the chemical structure of CNLDHAP70 composite was stable during the photocatalytic process (Fig. 7d). The chemical states of CNLDHAP70 after reaction were also investigated by XPS (Fig. 8a). The peaks at 576.9 and 586.9 eV contributed to the typical binding-energy peaks of Cr(III) (Wu et al. Reference Wu, Wang, Du, Li, Yang and Jia2015), which verified the successful reduction of Cr(VI). Meanwhile, the weak peak at the binding energy of 581.8 eV was due to the Cr(VI) on the surface of CNLDHAP70 adsorbed by electrostatic interaction.
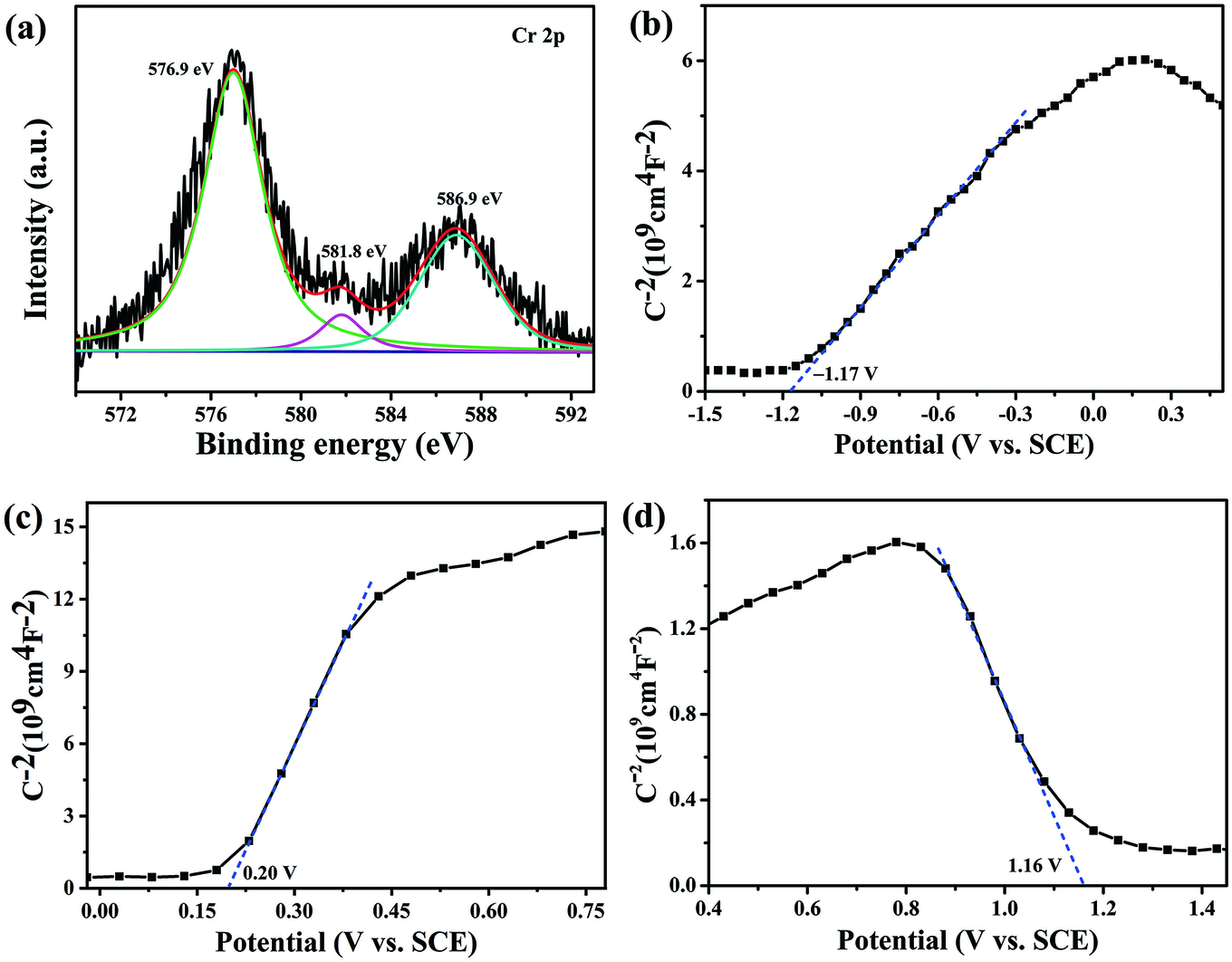
Fig. 8 a Cr 2p spectrum of the CNLDHAP70 composite after Cr(VI) reaction, Mott-Schottky plots of b g-C3N4, c Ag3PO4, and d LDH
Possible Mechanism for Cr(VI) Photocatalytic Reduction
In order to investigate the possible photocatalytic mechanism of Cr(VI) reduction, the band-edge positions were determined by the Mott-Schottky method. Both g-C3N4 and Ag3PO4 presented positive slopes in the linear region (Fig. 8b, c), suggesting an n-type semiconductor nature (Kang et al. Reference Kang, Cao, Zhang, Liu, Xu and Ye2013). Generally, the flat band potential (E fb) of n-type semiconductors is approximately equal to the conduction band (CB) potential (Xu et al. Reference Xu, Zhu, Zhu, Zhu, Liang, Zhu and He2017). Thus, the CB positions of g-C3N4 and Ag3PO4 were –1.17 and 0.20 V vs. SCE (equivalent to −0.93 and 0.44 V vs. NHE), respectively. The LDH showed a negative slope in the Mott-Schottky plot (Fig. 8d), indicating p-type semiconductor behavior. Due to the E fb of the p-type semiconductor being close to the valence band (VB) (Liu et al. Reference Liu, Chen, Li, Xu, Li, He and Lu2019b), the VB of LDH was 1.16 V vs. SCE (equivalent to 1.40 V vs. NHE). According to the equation E CB = E VB – E g (Hu et al. Reference Hu, Chen, Mo, Li, Xu, Li, He, Xu and Lu2019), the VB values of g-C3N4 and Ag3PO4 were 1.72 and 2.87 eV, and the CB of LDH was –0.70 eV.
Based on the results above, a potential multiple heterojunction mechanism was proposed to explain the enhanced photocatalytic activity of CNLDHAP composite photocatalyst. As shown in Fig. 9, the separation processes of photogenerated electron-hole pairs could be deduced to follow two possible paths. Under visible-light irradiation, g-C3N4, LDH, and Ag3PO4 were excited simultaneously to generate electron-hole pairs. With respect to charge-transfer Pathway I of the CNLDHAP composite, the electrons in the CB of g-C3N4 would migrate to the CB of LDH due to the potential difference between them. The holes in the VB of g-C3N4 would transfer first to the VB of LDH and then be eliminated by the electrons from the CB of Ag3PO4. Finally, the accumulated electrons in LDH could reduce Cr(VI) to Cr(III). The electron-transfer process of the CNLDHAP composite may also follow Pathway II, which is illustrated by the electrons in the CB of Ag3PO4 recombining with the holes in the VB of g-C3N4 and LDH, respectively, leaving the electrons in the CB of g-C3N4 and LDH to participate in the photocatalytic Cr(VI)-reduction reaction. Consequently, the synergy among those three semiconductors was effective in accelerating the spatial separation of photogenerated charge carriers and thus resulted in excellent photocatalytic performance of the ternary CNLDHAP composite photocatalyst.
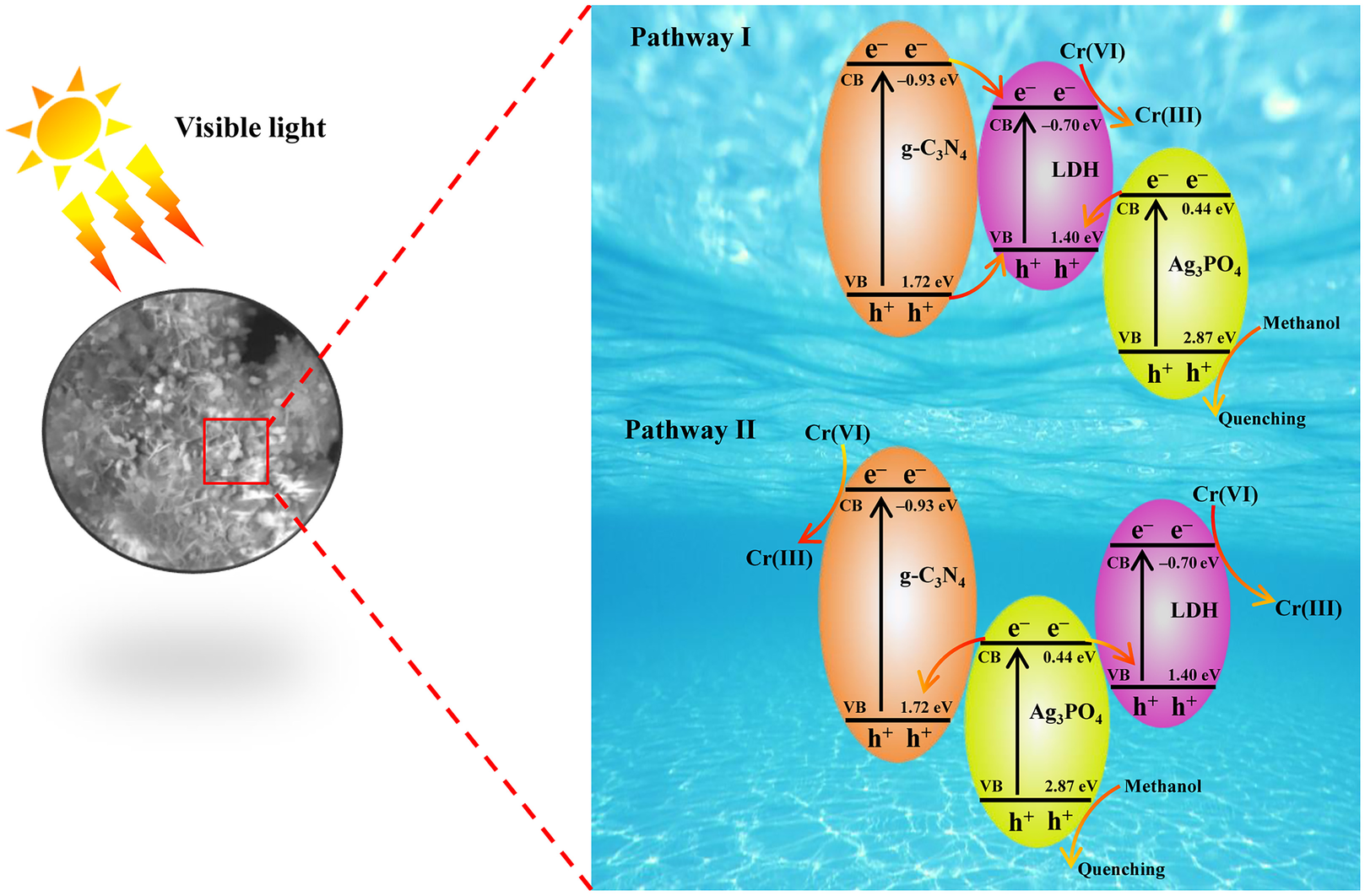
Fig. 9 Possible photocatalytic mechanism for the enhanced photocatalytic activity of the CNLDHAP composite photocatalyst
Conclusions
A multiple heterojunction system was constructed successfully using a two-step hydrothermal route. The photocatalytic activity of the CNLDHAP composite photocatalyst improved by 43.0–75.9% compared to the individual components, and the Cr(VI) reduction efficiency depended to a significant extent on the Ag3PO4 content. The optimum CNLDHAP70 showed the greatest photocatalytic activity for Cr(VI) reduction (96.0%), and the k value was ~15.67 and 11.75 times greater than that of g-C3N4 and LDH, respectively. CNLDHAP70 exhibited significant recycling stability: the Cr(VI) reduction efficiency remained at a high level after five cycles. The present study has provided new insights into the construction of multiple-heterojunction photocatalysts with improved separation efficiency of photogenerated carriers.
Supplementary Information
The online version contains supplementary material available at https://doi.org/10.1007/s42860-021-00121-0.
ACKNOWLEDGMENTS
This work was supported by the Key Research and Development Program of Hunan Province (2018SK20110).
Funding
Funding sources are as stated in the Acknowledgments.
Declarations
Conflict of Interest
The authors declare that they have no conflict of interest.











