Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Whitley, Heather D.
and
Smith, David E.
2004.
Free energy, energy, and entropy of swelling in Cs–, Na–, and Sr–montmorillonite clays.
The Journal of Chemical Physics,
Vol. 120,
Issue. 11,
p.
5387.
Greathouse, Jeffery A.
and
Cygan, Randall T.
2005.
Molecular dynamics simulation of uranyl(vi) adsorption equilibria onto an external montmorillonite surface.
Physical Chemistry Chemical Physics,
Vol. 7,
Issue. 20,
p.
3580.
Greathouse, Jeffery A.
Stellalevinsohn, Hannah R.
Denecke, Melissa A.
Bauer, Andreas
and
Pabalan, Roberto T.
2005.
Uranyl Surface Complexes in a Mixed-Charge Montmorillonite: Monte Carlo Computer Simulation and Polarized XAFS Results.
Clays and Clay Minerals,
Vol. 53,
Issue. 3,
p.
278.
Sutton, Rebecca
Sposito, Garrison
Diallo, Mamadou S.
and
Schulten, Hans-Rolf
2005.
Molecular simulation of a model of dissolved organic matter.
Environmental Toxicology and Chemistry,
Vol. 24,
Issue. 8,
p.
1902.
Smith, David E.
Wang, Yu
Chaturvedi, A.
and
Whitley, Heather D.
2006.
Molecular Simulations of the Pressure, Temperature, and Chemical Potential Dependencies of Clay Swelling.
The Journal of Physical Chemistry B,
Vol. 110,
Issue. 40,
p.
20046.
Cygan, Randall T.
Stevens, Caroline T.
Puls, Robert W.
Yabusaki, Steven B.
Wauchope, Robert D.
McGrath, Christian J.
Curtis, Gary P.
Siegel, Malcolm D.
Veblen, Linda A.
and
Turner, David R.
2007.
Research Activities at U.S. Government Agencies in Subsurface Reactive Transport Modeling.
Vadose Zone Journal,
Vol. 6,
Issue. 4,
p.
805.
Cygan, Randall T.
Greathouse, Jeffery A.
Heinz, Hendrik
and
Kalinichev, Andrey G.
2009.
Molecular models and simulations of layered materials.
Journal of Materials Chemistry,
Vol. 19,
Issue. 17,
p.
2470.
Roques, Jérôme
Veilly, Edouard
and
Simoni, Eric
2009.
Periodic Density Functional Theory Investigation of the Uranyl Ion Sorption on Three Mineral Surfaces: A Comparative Study.
International Journal of Molecular Sciences,
Vol. 10,
Issue. 6,
p.
2633.
Kier, Lemont B.
Cheng, Cho‐Kun
and
Nelson, Jean D.
2009.
Models of Solute Aggregation Using Cellular Automata.
Chemistry & Biodiversity,
Vol. 6,
Issue. 3,
p.
396.
Striolo, Alberto
2011.
From Interfacial Water to Macroscopic Observables: A Review.
Adsorption Science & Technology,
Vol. 29,
Issue. 3,
p.
211.
Joseph, C.
Schmeide, K.
Sachs, S.
Brendler, V.
Geipel, G.
and
Bernhard, G.
2011.
Sorption of uranium(VI) onto Opalinus Clay in the absence and presence of humic acid in Opalinus Clay pore water.
Chemical Geology,
Vol. 284,
Issue. 3-4,
p.
240.
Ebrahimi, Davoud
Pellenq, Roland J.-M.
and
Whittle, Andrew J.
2012.
Nanoscale Elastic Properties of Montmorillonite upon Water Adsorption.
Langmuir,
Vol. 28,
Issue. 49,
p.
16855.
Lectez, Sébastien
Roques, Jérôme
Salanne, Mathieu
and
Simoni, Eric
2012.
Car-Parrinello molecular dynamics study of the uranyl behaviour at the gibbsite/water interface.
The Journal of Chemical Physics,
Vol. 137,
Issue. 15,
Yang, W.
and
Zaoui, A.
2013.
Uranyl adsorption on (001) surfaces of kaolinite: A molecular dynamics study.
Applied Clay Science,
Vol. 80-81,
Issue. ,
p.
98.
Kerisit, Sebastien
and
Liu, Chongxuan
2013.
Structure, Kinetics, and Thermodynamics of the Aqueous Uranyl(VI) Cation.
The Journal of Physical Chemistry A,
Vol. 117,
Issue. 30,
p.
6421.
Bohinc, Klemen
Reščič, Jurij
Dufreche, Jean-Francois
and
Lue, Leo
2013.
Recycling of Uranyl from Contaminated Water.
The Journal of Physical Chemistry B,
Vol. 117,
Issue. 37,
p.
10846.
Geckeis, Horst
Lützenkirchen, Johannes
Polly, Robert
Rabung, Thomas
and
Schmidt, Moritz
2013.
Mineral–Water Interface Reactions of Actinides.
Chemical Reviews,
Vol. 113,
Issue. 2,
p.
1016.
Kerisit, Sebastien
and
Liu, Chongxuan
2014.
Molecular Dynamics Simulations of Uranyl and Uranyl Carbonate Adsorption at Aluminosilicate Surfaces.
Environmental Science & Technology,
Vol. 48,
Issue. 7,
p.
3899.
Amarasinghe, Priyanthi M.
Anandarajah, A.
and
Ghosh, Pijush
2014.
Molecular dynamic study of capillary forces on clay particles.
Applied Clay Science,
Vol. 88-89,
Issue. ,
p.
170.
Molinari, Marco
Brukhno, Andrey V.
Parker, Stephen C.
and
Spagnoli, Dino
2016.
Molecular Modeling of Geochemical Reactions.
p.
33.
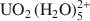 complexes translate within the clay pore through a jump diffusion process, and that first-shell water molecules are exchangeable and interchangeable.
complexes translate within the clay pore through a jump diffusion process, and that first-shell water molecules are exchangeable and interchangeable.

