No CrossRef data available.
Article contents
Meifuite, A New Ferrous Phyllosilicate Mineral With Modulated Tetrahedral Sheets Similar to Minnesotaite
Published online by Cambridge University Press: 01 January 2024
Abstract
A new ferrous phyllosilicate, meifuite, has been discovered in the Yinachang Fe-Cu-REE (rare-earth element) deposit in China. The structural formula, calculated using averaged electron probe microanalysis (EPMA) results, is K0.72Na0.20(Fe5.56Mg0.31Mn0.13)Σ6.00(Si6.95Al1.04)Σ7.99O18.84(OH)4.84 Cl1.33, with an ideal formula of KFe6(AlSi7)O19(OH)4Cl2. The structure of meifuite has a P1¯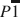 space group symmetry, with unit-cell parameters of a = 22.7773(13) Å, b = 9.5553(5) Å, c =14.3282(8) Å, α = 99.258(4)°, β = 136.750(3)°, γ = 89.899(4)°, Z = 2, and V = 2077.9(2) Å3. Meifuite has a strip-modulated 2:1 layer (T–O–T) structure similar to that of minnesotaite. About 1/8 of the tetrahedra in the T sheet are occupied by Al instead of Si, and the interlayer cavities are partially occupied by K and Na. Some of the OH sites in the octahedral sheet in the layer structure are fully or partially substituted by Cl, which is apparently the primary reason for the meifuite structure being more stable than stilpnomelane, the most common ferrous layer silicate mineral found at similar temperature and pressure conditions. An updated, more accurate structure model of minnesotaite is also provided for comparison with the meifuite structure. The mineral is named after Meifu Zhou in honor of his outstanding contributions to the field of economic geology.
space group symmetry, with unit-cell parameters of a = 22.7773(13) Å, b = 9.5553(5) Å, c =14.3282(8) Å, α = 99.258(4)°, β = 136.750(3)°, γ = 89.899(4)°, Z = 2, and V = 2077.9(2) Å3. Meifuite has a strip-modulated 2:1 layer (T–O–T) structure similar to that of minnesotaite. About 1/8 of the tetrahedra in the T sheet are occupied by Al instead of Si, and the interlayer cavities are partially occupied by K and Na. Some of the OH sites in the octahedral sheet in the layer structure are fully or partially substituted by Cl, which is apparently the primary reason for the meifuite structure being more stable than stilpnomelane, the most common ferrous layer silicate mineral found at similar temperature and pressure conditions. An updated, more accurate structure model of minnesotaite is also provided for comparison with the meifuite structure. The mineral is named after Meifu Zhou in honor of his outstanding contributions to the field of economic geology.
- Type
- Article
- Information
- Copyright
- Copyright © Clay Minerals Society 2021


