Article contents
Infrared Study of CO2 Incorporation into Pyrophyllite [Al2Si4O10(OH)2] during Dehydroxylation
Published online by Cambridge University Press: 01 January 2024
Abstract
We report infrared spectroscopic observations of the incorporation of CO2 into pyrophyllite that has been heated between 200°C and 1250°C for periods of 15 min, 1 h and 5 days. The presence of CO2 is characterized by the ν3 band of CO2 near 2347 cm−1, detectable in samples in which dehydroxylation has commenced after heating above 450°C. With increasing temperature, the CO2 signal becomes more intense. The signal reaches its maximum intensity near 800°C with an annealing time of 15 min. Further heating leads to a decrease in the CO2 signal and the occurrence of an extra signal near 2156 cm−1 that implies the presence of CO. The process is characterized by significant time-dependence, indicating its kinetic nature. The peak positions of CO2 signals show systematic variations with temperature. Our results suggest that the CO2 molecule is associated with the local structure rather than being present as free gaseous CO2, and that the local structure of pyrophyllite is gradually modified during high-temperature treatments. However, no signals related to carbonate molecules ((CO32−)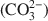 ) were detected. The results suggest that CO2 or other carbon-based molecules may diffuse into some clay minerals during dehydroxylation and may become altered due to structural modifications at high temperatures. This may have significance for possible CO2 sequestration in shales and clay formations.
) were detected. The results suggest that CO2 or other carbon-based molecules may diffuse into some clay minerals during dehydroxylation and may become altered due to structural modifications at high temperatures. This may have significance for possible CO2 sequestration in shales and clay formations.
- Type
- Research Article
- Information
- Copyright
- Copyright © 2003, The Clay Minerals Society
References
- 9
- Cited by


