Article contents
Dissolution of Brucite on the (001) Surface at Neutral pH: in situ Atomic Force Microscopy Observations
Published online by Cambridge University Press: 01 January 2024
Abstract
The dissolution of brucke, Mg(OH)2, on the (001) surface was investigated using in situ atomic force microscopy in solutions at near-neutral pH. Dissolution proceeded by the formation of crystallographically oriented triangular etch pits with monolayer step and expansion of the pits. The sides of the triangle are parallel to the [100], [110] and [010] directions of the brucite structure, and the orientation of lines from the center of the triangle to the three apices are along the [210], [1¯10]$[\bar 110]$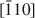 and [1¯2¯0]$[\bar 1\bar 20]$
and [1¯2¯0]$[\bar 1\bar 20]$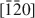 directions. This orientation may produce pit edges where OH groups coordinate to two Mg2+. Although triangular etch pits with monolayer depth formed mostly at random on the (001) surface, concentric pits penetrating several layers were also observed. Etch pits with spiral steps were rarely observed. Coalescence of the pits resulted in stranded terraces that diminished in size rapidly and formed a rounded irregular form. The step-retreat velocity around the triangular pit is 0.015–0.04 nm/s at pH 5–8. The retreat velocity around the stranded terraces was about three times more rapid than that around the triangular etch pits.
directions. This orientation may produce pit edges where OH groups coordinate to two Mg2+. Although triangular etch pits with monolayer depth formed mostly at random on the (001) surface, concentric pits penetrating several layers were also observed. Etch pits with spiral steps were rarely observed. Coalescence of the pits resulted in stranded terraces that diminished in size rapidly and formed a rounded irregular form. The step-retreat velocity around the triangular pit is 0.015–0.04 nm/s at pH 5–8. The retreat velocity around the stranded terraces was about three times more rapid than that around the triangular etch pits.
- Type
- Research Article
- Information
- Copyright
- Copyright © 2006, The Clay Minerals Society
References
- 8
- Cited by


