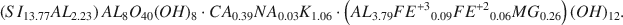Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
MOJARES, Edwin M.
TOMITA, Katsutoshi
and
KAWANO, Motoharu
2000.
Characterization of interstratified minerals associated with argillic alteration in steam-heated environment at Solo, Mabini, Philippines..
JOURNAL OF MINERALOGY, PETROLOGY AND ECONOMIC GEOLOGY,
Vol. 95,
Issue. 2,
p.
37.
Stastny, Martin
2014.
Argillization of topaz-bearing granites in the Hub stock, Horni Slavkov-Krasno Sn-W ore district (Bohemian Massif, Czech Republic).
Acta Geodynamica et Geomaterialia,
p.
255.
Billon, Sophie
Patrier, Patricia
Beaufort, Daniel
Sardini, Paul
and
Wattinne-Morice, Aurélia
2016.
Occurrence of tosudite in the Guezouman, Tarat and Tchirezrine 2 formations, hosts of uranium deposits in Niger (Tim Mersoï basin).
Clay Minerals,
Vol. 51,
Issue. 4,
p.
635.
Ogorodova, L. P.
Vigasina, M. F.
Vlasov, E. A.
Mel’chakova, L. V.
Krupskaya, V. V.
and
Spiridonov, E. M.
2018.
Li-Tosudite: X-Ray Diffraction, IR Spectroscopic, Thermal, and Thermochemical Studies.
Geochemistry International,
Vol. 56,
Issue. 3,
p.
234.
Cannata, Chiara Benedetta
Godbert, Nicolas
De Rosa, Rosanna
Aiello, Iolinda
Giorno, Eugenia
Croce, Alessandro
and
Bloise, Andrea
2023.
From Stromboli ashes to corrensite by hydrothermal synthesis: Hydrogeological inputs into Mars history.
Applied Clay Science,
Vol. 242,
Issue. ,
p.
107029.