Article contents
Crystal Structures of Biotite at High Temperatures and of Heat-Treated Biotite using Neutron Powder Diffraction
Published online by Cambridge University Press: 01 January 2024
Abstract
The crystal structure of biotite-1 M from Bancroft, Ontario, with the formula: (K1.96Na0.13Ca0.01)(Mg3.15Fe2.592+Ti0.17Mn0.09)(Si5.98Al1.92Ti0.10)O20[(OH)1.47F1.98]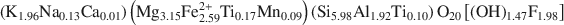 , was determined by Rietveld refinement using high-resolution neutron powder diffraction at in situ temperatures ranging from 20 to 900°C. The room-temperature structure of the samples heated to between 400 and 900°C using an electric furnace in air was also refined. The crystal structures were refined to an RP of 2.98 — 5.06% and Rwp of 3.84–6.77%. For the in situ heating experiments in a vacuum, the unit-cell dimensions increased linearly to 600°C. The linear expansion coefficient for the c axis was 1.65 × 10−5°C−1, while those for the a and b dimensions were 4.44 × 10−6°C−1 and 5.21 × 10−6°C−1, respectively. Accordingly, the increase in the unit-cell volume up to 600 C occurred mainly along the c axis, resulting from the expansion in the K coordination sphere along that direction. Results for all K−O bonds were analyzed in terms of the lattice component and an inner component of the structural strain. The ditrigonal distortion decreased (3.76 at 20°C to 1.95 at 600°C) with temperature, because the shorter bonds expanded and the longer bonds contracted. The increase in the interlayer separation and the decrease in the interlayer octahedral flattening angle confirmed that the c-dominated expansion occurred in the interlayer region. In the case of the ex situ-heated samples, the cell dimensions decreased sharply at temperatures over 400 C. The octahedral sheet thickness and mean <M−O> distance decreased linearly due to oxidation of octahedral Fe. However, the interlayer separation and mean <K−O> distance decreased at temperatures over 400°C. At 400°C, dehydroxylation began to increase and interlayer regions became more constricted. The overall cell parameters decreased rapidly with increasing temperatures due to dehydroxylation. The large inner strain components in the K−O bonds also resulted in an increase in the considerable ditrigonal distortion (3.57° at 400°C to 6.15° at 900°C).
, was determined by Rietveld refinement using high-resolution neutron powder diffraction at in situ temperatures ranging from 20 to 900°C. The room-temperature structure of the samples heated to between 400 and 900°C using an electric furnace in air was also refined. The crystal structures were refined to an RP of 2.98 — 5.06% and Rwp of 3.84–6.77%. For the in situ heating experiments in a vacuum, the unit-cell dimensions increased linearly to 600°C. The linear expansion coefficient for the c axis was 1.65 × 10−5°C−1, while those for the a and b dimensions were 4.44 × 10−6°C−1 and 5.21 × 10−6°C−1, respectively. Accordingly, the increase in the unit-cell volume up to 600 C occurred mainly along the c axis, resulting from the expansion in the K coordination sphere along that direction. Results for all K−O bonds were analyzed in terms of the lattice component and an inner component of the structural strain. The ditrigonal distortion decreased (3.76 at 20°C to 1.95 at 600°C) with temperature, because the shorter bonds expanded and the longer bonds contracted. The increase in the interlayer separation and the decrease in the interlayer octahedral flattening angle confirmed that the c-dominated expansion occurred in the interlayer region. In the case of the ex situ-heated samples, the cell dimensions decreased sharply at temperatures over 400 C. The octahedral sheet thickness and mean <M−O> distance decreased linearly due to oxidation of octahedral Fe. However, the interlayer separation and mean <K−O> distance decreased at temperatures over 400°C. At 400°C, dehydroxylation began to increase and interlayer regions became more constricted. The overall cell parameters decreased rapidly with increasing temperatures due to dehydroxylation. The large inner strain components in the K−O bonds also resulted in an increase in the considerable ditrigonal distortion (3.57° at 400°C to 6.15° at 900°C).
- Type
- Research Article
- Information
- Copyright
- Copyright © 2003, The Clay Minerals Society
References
- 17
- Cited by


