Article contents
Controlled Polymerization of Metanilic Anion within the Interlayer of NiAl Layered Double Hydroxide
Published online by Cambridge University Press: 01 January 2024
Abstract
The controlled chemical oxidative polymerization of metanilic anion (m-NH2C6H4SO3−)$(m{\rm{ - N}}{{\rm{H}}_2}{{\rm{C}}_6}{{\rm{H}}_4}{\rm{SO}}_3^ - )$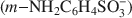 within the interlayer of NiAl layered double hydroxide was performed using, for the first time, ammonium persulfate as the oxidizing agent. The quantity of oxidizing agent required for control of the interlayer polymerization was investigated systematically and it was found that interleaved polyaniline sulfonic (PANIS) was present in different oxidation states and protonation levels when different quantities of external oxidizing agents were added. A mechanism for the oxidative polymerization of metanilic anion in NiAl layered double hydroxide is proposed, based on the intercalation of the oxidizing agent and the interlayer polymerization of monomer. The resulting PANIS/NiAl LDH composites were characterized by powder X-ray diffraction, ultraviolet-visible absorption spectra, Fourier transform infrared and X-ray photoelectron spectroscopy.
within the interlayer of NiAl layered double hydroxide was performed using, for the first time, ammonium persulfate as the oxidizing agent. The quantity of oxidizing agent required for control of the interlayer polymerization was investigated systematically and it was found that interleaved polyaniline sulfonic (PANIS) was present in different oxidation states and protonation levels when different quantities of external oxidizing agents were added. A mechanism for the oxidative polymerization of metanilic anion in NiAl layered double hydroxide is proposed, based on the intercalation of the oxidizing agent and the interlayer polymerization of monomer. The resulting PANIS/NiAl LDH composites were characterized by powder X-ray diffraction, ultraviolet-visible absorption spectra, Fourier transform infrared and X-ray photoelectron spectroscopy.
- Type
- Research Article
- Information
- Copyright
- Copyright © 2006, The Clay Minerals Society
References
- 5
- Cited by


