Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Inoue, Atsuyuki
Kurokawa, Kyohei
and
Hatta, Tamao
2010.
Application of Chlorite Geothermometry to Hydrothermal Alteration in Toyoha Geothermal System, Southwestern Hokkaido, Japan.
Resource Geology,
Vol. 60,
Issue. 1,
p.
52.
Guggenheim, Stephen
2011.
Layered Mineral Structures and their Application in Advanced
Technologies.
p.
73.
Kameda, Jun
Ujiie, Kohtaro
Yamaguchi, Asuka
and
Kimura, Gaku
2011.
Smectite to chlorite conversion by frictional heating along a subduction thrust.
Earth and Planetary Science Letters,
Vol. 305,
Issue. 1-2,
p.
161.
NITTA, Miyuki
and
INOUE, Atsuyuki
2011.
Carbon and Oxygen Isotope Compositions of Calcite and Rhodochrosite from Geothermal Exploratory Drills TH‐4 and TH‐6 near the Toyoha Deposit, Hokkaido, Japan.
Resource Geology,
Vol. 61,
Issue. 2,
p.
159.
Wang, Hejing
Ma, Yongsheng
Zhou, Jian
and
Xu, Tingjing
2012.
Diagenesis and very low grade metamorphism in a 7,012 m-deep well Hongcan 1, eastern Tibetan plateau.
Swiss Journal of Geosciences,
Vol. 105,
Issue. 2,
p.
249.
Leclère, Henri
Buatier, Martine
Charpentier, Delphine
Sizun, Jean-Pierre
Labaume, Pierre
and
Cavailhes, Thibault
2012.
Formation of phyllosilicates in a fault zone affecting deeply buried arkosic sandstones: their influence on petrophysic properties (Annot sandstones, French external Alps).
Swiss Journal of Geosciences,
Vol. 105,
Issue. 2,
p.
299.
Lanari, Pierre
Guillot, Stéphane
Schwartz, Stéphane
Vidal, Olivier
Tricart, Pierre
Riel, Nicolas
and
Beyssac, Olivier
2012.
Diachronous evolution of the alpine continental subduction wedge: Evidence from P–T estimates in the Briançonnais Zone houillère (France – Western Alps).
Journal of Geodynamics,
Vol. 56-57,
Issue. ,
p.
39.
Ferreiro Mählmann, Rafael
Nieto, Fernando
Bozkaya, Ömer
Potel, Sébastien
and
Türkmenoğlu, Asuman Günal
2012.
Preface: clay mineral diagenesis and very low-grade metamorphic processes. Proceedings of the 2011 Frey–Kübler symposium.
Swiss Journal of Geosciences,
Vol. 105,
Issue. 2,
p.
117.
Lacroix, B.
Charpentier, D.
Buatier, M.
Vennemann, T.
Labaume, P.
Adatte, T.
Travé, A.
and
Dubois, M.
2012.
Formation of chlorite during thrust fault reactivation. Record of fluid origin and P–T conditions in the Monte Perdido thrust fault (southern Pyrenees).
Contributions to Mineralogy and Petrology,
Vol. 163,
Issue. 6,
p.
1083.
Buatier, Martine D.
Lacroix, Brice
Labaume, Pierre
Moutarlier, Virginie
Charpentier, Delphine
Sizun, Jean Pierre
and
Travé, Anna
2012.
Microtextural investigation (SEM and TEM study) of phyllosilicates in a major thrust fault zone (Monte Perdido, southern Pyrenees): impact on fault reactivation.
Swiss Journal of Geosciences,
Vol. 105,
Issue. 2,
p.
313.
Grosch, E. G.
Vidal, O.
Abu-Alam, T.
and
McLoughlin, N.
2012.
P-T Constraints on the Metamorphic Evolution of the Paleoarchean Kromberg Type-Section, Barberton Greenstone Belt, South Africa.
Journal of Petrology,
Vol. 53,
Issue. 3,
p.
513.
Kameda, Jun
Hina, Shoko
Kobayashi, Kyoko
Yamaguchi, Asuka
Hamada, Yohei
Yamamoto, Yuzuru
Hamahashi, Mari
and
Kimura, Gaku
2012.
Silica diagenesis and its effect on interplate seismicity in cold subduction zones.
Earth and Planetary Science Letters,
Vol. 317-318,
Issue. ,
p.
136.
Bourdelle, Franck
Parra, Teddy
Chopin, Christian
and
Beyssac, Olivier
2013.
A new chlorite geothermometer for diagenetic to low-grade metamorphic conditions.
Contributions to Mineralogy and Petrology,
Vol. 165,
Issue. 4,
p.
723.
Wang, H.
Rahn, M.
Zhou, J.
and
Tao, X.
2013.
Tectonothermal evolution of the Triassic flysch in the Songpan–Garzê orogen, eastern Tibetan plateau.
Tectonophysics,
Vol. 608,
Issue. ,
p.
505.
Bourdelle, Franck
Benzerara, Karim
Beyssac, Olivier
Cosmidis, Julie
Neuville, Daniel R.
Brown, Gordon E.
and
Paineau, Erwan
2013.
Quantification of the ferric/ferrous iron ratio in silicates by scanning transmission X-ray microscopy at the Fe L2,3 edges.
Contributions to Mineralogy and Petrology,
Vol. 166,
Issue. 2,
p.
423.
Livi, Kenneth J.T.
and
Abad, Isabel
2013.
Minerals at the Nanoscale.
p.
217.
Rogers, A. J.
Kolb, J.
Meyer, F. M.
and
Vennemann, T.
2013.
Two stages of gold mineralization at Hutti mine, India.
Mineralium Deposita,
Vol. 48,
Issue. 1,
p.
99.
Ossa Ossa, Frantz
Hofmann, Axel
Vidal, Olivier
Kramers, Jan D.
Agangi, Andrea
Belyanin, Georgy A.
and
Mayaga-Mikolo, Francis
2014.
Hydrothermal clay mineral formation in the uraniferous Paleoproterozoic FA Formation, Francevillian basin, Gabon.
Precambrian Research,
Vol. 246,
Issue. ,
p.
134.
Leclère, Henri
Lacroix, Brice
and
Fabbri, Olivier
2014.
Fault mechanics at the base of the continental seismogenic zone: Insights from geochemical and mechanical analyses of a crustal-scale transpressional fault from the Argentera crystalline massif, French–Italian Alps.
Journal of Structural Geology,
Vol. 66,
Issue. ,
p.
115.
Torgersen, E.
and
Viola, G.
2014.
Structural and temporal evolution of a reactivated brittle–ductile fault – Part I: Fault architecture, strain localization mechanisms and deformation history.
Earth and Planetary Science Letters,
Vol. 407,
Issue. ,
p.
205.
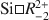 exchange at low-T conditions while they are controlled by Tschermak exchange at higher temperatures. The decreasing number of vacancies with temperature are poorer in Fe-rich than in Fe-poor chlorites. Furthermore, the ordered-site occupation of cations and vacancies in trioctahedral chlorite occurs concomitantly with the compositional changes ruled by increasing temperature conditions.
exchange at low-T conditions while they are controlled by Tschermak exchange at higher temperatures. The decreasing number of vacancies with temperature are poorer in Fe-rich than in Fe-poor chlorites. Furthermore, the ordered-site occupation of cations and vacancies in trioctahedral chlorite occurs concomitantly with the compositional changes ruled by increasing temperature conditions.

