Introduction
The detrimental impact of soil and water pollution, originating from industrial waste water, contaminated sites, tailings, and farmland, has become a focal point of global concern. Heavy metals, in contrast to organic pollutants, are not subject to decomposition and have a propensity for migration and dispersion through groundwater. This process culminates in the accumulation of these metals in the human body, giving rise to severe endemic diseases (Ji et al., Reference Ji, Kim, Lee, Park, Kwon, Cheong, Jang, Kim, Yu, Kim, Lee, Yang, Jhung, Yang, Paek, Hong and Choi2013; Bai and Zhao, Reference Bai and Zhao2020; Kharazi et al., Reference Kharazi, Leili, Khazaei, Alikhani and Shokoohi2021). Consequently, strategies for the extraction and stabilization of heavy metal pollutants from water and soil are critical for the safeguarding of ecosystem security (Järup, Reference Järup2003).
In response to the growing concern about heavy metal pollution, a variety of potential remediation technologies have been developed (Chai et al., Reference Chai, Cheun, Kumar, Mubashir, Majeed, Banat, Ho and Show2021). These include in situ soil remediation methods such as biological treatment (Farrell and Jones, Reference Farrell and Jones2009), stabilization/solidification (Xu et al., Reference Xu, Fu, Wang, Shi and Guo2021), aqueous flushing (Atteia et al., Reference Atteia, Del Campo Estrada and Bertin2013), and permeable reactive barrier (Faisal et al., Reference Faisal, Sulaymon and Khaliefa2018). Additionally, liquid phase remediation technologies for industrial effluents and polluted watercourses (Kurniawan et al., Reference Kurniawan, Chan, Lo and Babel2006; Xu et al., Reference Xu, Zhang, Li and Huang2022), encompass ion exchange (Da̧browski et al., Reference Da̧browski, Hubicki, Podkościelny and Robens2004), membrane separation (Abdullah et al., Reference Abdullah, Yusof, Lau, Jaafar and Ismail2019), adsorption (Han et al., Reference Han, Rafiq, Zhou, Xu, Mašek and Li2019), flocculation and biological treatment (Chipasa, Reference Chipasa2003). However, despite the strides made in technological innovation, these methods have revealed limitations in practical applications. Notably, biological treatment is constrained by the depth of contaminated soil, as it exclusively accumulates heavy metals in shallow soil through plants or bacteria. Stabilization/solidification methods, while effective in preventing the migration of heavy metals or inhibiting their activity (toxicity) through repair materials (stabilizers), grapple with the persistence of metals in the soil. The potential re-release of metals when the repair agent fails and soil salinization with the application of alkaline stabilizers like cement and lime. The aqueous flushing method, designed to remove contaminants from contaminated soil through injection-pumping, necessitates the subsequent centralized treatment of the rinsed liquid by efficient adsorbents to capture heavy metals. The permeable reactive barrier, a widely employed engineering technique, mandates coordination measures to impede the outward diffusion of contaminants on the site. This technique is commonly integrated with adsorption-reaction materials to effectively capture or stabilize contaminants. Even the membrane separation method depends on adsorbents after forming a side concentrate. Consequently, the utilization of chemical or physical adsorption for the efficient removal of contaminants presents a promising solution in the realm of soil-water pollution treatment.
Recent studies highlight activated carbon (Deliyanni et al., Reference Deliyanni, Kyzas, Triantafyllidis and Matis2015), natural or modified biochar (Tan et al., Reference Tan, Liu, Zeng, Wang, Hu, Gu and Yang2015; Wang et al., Reference Wang, Wang, Ma, Tankpa, Bai, Guo and Wang2019), and clay minerals (Uddin, Reference Uddin2017) as primary adsorbents for heavy metal removal. Among these, clay minerals, especially bentonite, outperform other commercial adsorbents due to their superior adsorption performance, cost-effectiveness, and widespread availability. Bentonite, primarily composed of montmorillonite, boasts an exceptionally high specific surface area and a lamellar structure (Yu et al., Reference Yu, Liao, Cai and Yu2019a). The surplus surface negative charge of montmorillonite allows bentonite to adsorb heavy metals through both surface adsorption and ion exchange concurrently. This property has led to its extensive use in various adsorption scenarios and permeable reactive barrier materials (Yu et al., Reference Yu, Yang, Wu, Jiang, Liao and Deng2021). However, natural bentonite is limited in industrial applications for its low quality and easy desorption (Han et al., Reference Han, Rafiq, Zhou, Xu, Mašek and Li2019). Consequently, the modification of bentonite to enhance its adsorption capacity presents a promising avenue with broad application prospects.
Various techniques have been explored to modify bentonite, including acid activation (Malamis and Katsou, Reference Malamis and Katsou2013; Fazlali et al., Reference Fazlali, Mahjoub and Aghayan2019), calcination (Aytas et al., Reference Aytas, Yurtlu and Donat2009), pillared (Manohar et al., Reference Manohar, Noeline and Anirudhan2005; Tomul, Reference Tomul2012), surfactant treatment (Ghiaci et al., Reference Ghiaci, Kalbasi and Abbaspour2007; Díaz-Nava et al., Reference Díaz-Nava, Olguín and Solache-Ríos2012; Xie et al., Reference Xie, Wu, Yu, Yan, Masum, Cai and Chen2023b), activator loading (Cai et al., Reference Cai, Yu, Yu, Wu, Li and Yu2019; Shao et al., Reference Shao, Yu, Zhou, Cai and Yu2018; Yu et al., Reference Yu, Lv, Zhou, Cai, Zha and Yu2019b; Li et al., Reference Li, Yu, Wu, Cai and Zha2020; Yu et al., Reference Yu, Shao, Sun and Yu2020) and polymer modification (Souza and Nascimento, Reference Souza and Nascimento2008; Kotal and Bhowmick, Reference Kotal and Bhowmick2015; Wang et al., Reference Wang, Chen, Xie, Zhang and Zhan2016; Xie et al., Reference Xie, Chen, Shi, Zheng, Chen and Yan2023a). Acid activation can unclog pore channels, increase the specific surface area, and enhance the adsorption activity of metal ions by dissolving natural cementing impurities in the bentonite, leading to hydrogen ion substitution at the adsorption site. Calcination, while increasing the specific surface area by gasifying strongly bound water, requires high temperature and energy consumption. Pillaring, although it extends the distance between bentonite layers, destroys the free property between layers, making montmorillonite’s structure similar to zeolite and reducing swelling properties. Surfactant treatment follows the cation exchange principle to increase bentonite’s organic adsorption capacity. Yet, the difficulty in replacing the surfactants introduced with metal cations reduces its exchange adsorption capacity for heavy metals. Polymer-modified bentonite can change the specific surface area, pore volume, and functional group content of soil particles (such as -NH2, -COOH, and -OH) due to its polymer structure characteristics. The organic chains introduced are also expected to remove heavy metals and organic pollutants simultaneously (Han et al., Reference Han, Rafiq, Zhou, Xu, Mašek and Li2019), which has become a popular direction for bentonite modification. However, there are few studies on the co-adsorption mechanism of polymers and clays, especially desorption. This is very important for understanding the adsorption mechanism of heavy metals and the recycling of adsorbents.
In addition, most heavy metals exist as positively charged ions, prompting a common strategy to enhance bentonite’s adsorption capacity through the introduction of negative charge or polar functionalities. However, challenges arise due to disparities in hydration and swelling, hindering the formation of stable composite materials between polymers and clay minerals. In this study, sodium polyacrylate, recognized for its high efficiency and abundant negative charges, was grafted onto bentonite using an organosilane coupling agent. This nanocomposite synthesis aimed to investigate the adsorption and desorption characteristics of lead ions, representing cationic metal pollutants, on the polycarboxylate-grafted bentonite (SAPAS-Bentonite). The results provide valuable insights into the potential applications and adsorption mechanisms of this sodium polyacrylate modified clay, paving the way for future advancements in this field.
Materials and Methods
Materials and reagents
The raw bentonite used in this study was sourced from Yixing Jintai Geotechnical Materials Co., Ltd, located in Jiangsu, China. Before utilization, the bentonite underwent a purification process involving sieving through a 200-mesh (75 μm) sieve and subsequent drying in an oven at 105°C for 48 h. The purified bentonite was stored in a polypropylene container within a desiccator. Mineral analysis indicated that the raw bentonite consists of montmorillonite (45%), quartz (24%), heulandite (15%), and calcite (15%), with a cation exchange capacity (CEC) of 66.08 cmol kg–1. All chemicals used in the experiments were procured from Shanghai Aladdin Biochemical Technology Co., Ltd, and met analytical purity standards. Solutions for the experiments were prepared using deionized water.
Methods
Synthesis of SAPAS-Bentonite
SAPAS-Bentonite was synthesized through solution polymerization (Yu et al., Reference Yu, Liao, Cai and Yu2019a), employing raw bentonite, triethoxyvinylsilane, acrylic acid, and other auxiliary materials in the dosages depicted in Table 1. The synthesis consisted of two steps (Fig. 1): (1) the grafting of alkenylsilanol groups onto mineral surfaces in bentonite; and (2) the double bond polymerization of acrylic acid with alkene groups. Initially, triethoxyvinylsilane underwent hydrolysis in deionized water, followed by the addition of bentonite to form a slurry. This slurry was stirred continuously at 85°C for 60 min, facilitating the completion of grafting. Subsequently, N,N′-methylenebisacrylamide, and acrylic acid were introduced, followed by the addition of potassium persulfate to initiate polymerization after 30 min of stirring. Finally, the pH of the slurry was adjusted to 7 using sodium hydroxide, after which it was centrifuged, washed repeatedly, and dried at 105°C.

Figure 1. Flowchart of preparation of SAPAS-Bentonite.
Table 1. The raw materials and ratio of SAPAS-Bentonite

* The amount of NaOH used here is approximate.
Batch adsorption and desorption experiments
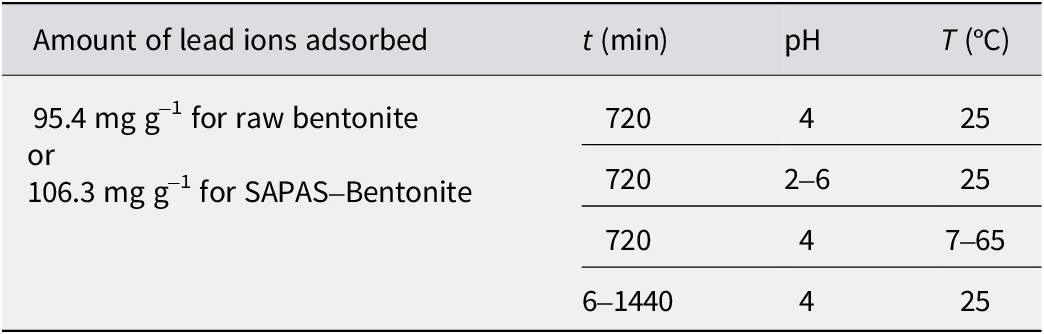
Both adsorption and desorption tests were conducted in batches. In the adsorption batch, 0.1 g of bentonite was added to 15 mL centrifuge tubes containing 10 mL of lead ions solution at a pre-determined concentration. The contents were mixed gently for a specific time at a designated temperature. The desorption process followed a similar procedure, using lead-adsorbed bentonite and a desorption reagent instead of initial bentonite. The lead-adsorbed raw bentonite absorbed 95.4 mg g–1 of lead ions, while SAPAS-Bentonite adsorbed 106.3 mg g–1. These adsorbed samples were dried, ground, and used for desorption experiments. For concentration-dependent desorption, the tubes were oven-dried after adsorption (without centrifugal separation), ground, and reused. Soil–water separation operations were carried out by centrifugation (5867×g using a Xiangyi H1850, Changsha, China) at 8000 r.p.m. for 3 min, excluding the acceleration phase. It is noteworthy that centrifugation time was included in dynamic timing analyses. The residual or dissociated metal ions before and after experiments were analyzed using a flame atomic absorption spectrophotometer (Shimadzu AA6880F, Kyoto, Japan). The effects of adsorbent initial concentration (ci), pH value, experimental temperature (T), and contact time (t) on the adsorption capacity were examined (Tables 2 and 3). pH was adjusted using 0.1 M and 0.01 M HCl or NaOH. Adsorption amounts were determined by comparing the concentration of lead ions before and after the adsorption or desorption process. Each experiment was conducted twice to ensure accuracy.
Table 2. Batch adsorption and experiments parameters

Table 3. Batch desorption and experiments parameters
Theoretical approach
The per cent adsorption (%) of lead ions was calculated using:
where c i is the initial concentration of the lead ions solution (mg L–1), and c e is the equilibrium concentration of the lead ions solution (mg L–1). Similarly, the adsorption capacity (q e, mg g–1) was calculated as:
where v is the volume of lead ions solution (mL) and m ads is the mass of the adsorbent (g).
In this study, equilibrium experimental data for the adsorption of lead ions on the composite were analyzed using the Langmuir and Freundlich isotherm models to describe the adsorption data. The Langmuir model (Langmuir, Reference Langmuir1918) is expressed as:
The model can be expressed in a linear form as:
where q m denotes the maximum adsorption capacity (mg g–1), and b is the Langmuir adsorption equilibrium constant (L mg–1), which is related to the heat of adsorption. This linear form proves useful for plotting and analyzing experimental data.
The expression of Freundlich model (Freundlich, Reference Freundlich1907), an empirical equipment describing adsorption to heterogeneous surface, is given as:
The model also can be expressed in the linear form as:
where F and n are the Freundlich coefficients; the former represents the adsorption capacity when the metal ion equilibrium concentration equals 1, and the latter represents the degree of dependence of the adsorption on the equilibrium concentration.
Kinetics for clay–metal interaction was adjusted in this study by applying the pseudo-first order kinetics and pseudo-second order kinetics equations (Ezzati, Reference Ezzati2020). The two models are discrete and are expressed as:
and
where q e and q t are the values for the adsorbed amount per unit mass at equilibrium and at any given time t, and k 1 and k 2 are discrete and the pseudo-first order and pseudo-second order adsorption rate constants.
Characterizations analysis
X-ray diffraction (XRD) analysis was conducted using a Bruker D8 Advance X-ray diffractometer (Karlsruhe, Germany), with CuKα radiation (λ = 1.5406 Å) at a scanning rate of 8° min–1 in the 2θ range from 5 to 75°. Thermogravimetric analysis (TGA) was performed using Perkin Elmer TGA instrument (Diamond TG-DTA, Waltham, MA, USA) at a heating rate of 10°C min–1 in an oxygen atmosphere. The microstructure of bentonites, post-adsorption at varying lead concentrations, was examined using scanning electron microscopy (SEM) with an FEI Nova NanoSEM 200 instrument (Hillsboro, OR, USA) at a magnification range of 5000–30,000X. The bentonite samples were evaluated according to the Chinese standard for bentonite (GB/T 20973-2020). Specifically, all exchangeable cations in the bentonite samples were replaced by Ba2+ using a BaCl2 solution to obtain pure Ba-bentonite. Subsequently, Ba-bentonite underwent a full exchange of Ba2+ to Mg2+ in a MgSO4 solution, as Ba2+ is completely precipitated by SO42–. Finally, the CEC value was determined by measuring the reduction of Mg2+ in the MgSO4 solution.
Results and Discussion
Preparation of SAPAS-Bentonite
The modified substance of polycarboxylate graft-coated bentonite represents the mineral modification of montmorillonite, the primary active mineral. The crystal edges of montmorillonite are abundant in hydroxyl groups such as Si-OH and Al-OH. Consequently, the silanol groups, generated by organosilane hydrolysis, can readily dehydrate and condense with the original hydroxyl structure of montmorillonite. This process enables the growth of triethoxyvinylsilane on the mineral surface. According to several studies on silicone adhesives (Pape, Reference Pape and Kutz2011; Stewart et al., Reference Stewart, Schlosser and Douglas2013), silicon hydroxyl groups appear capable of achieving chemical bonding, even in the absence of hydroxyl structural clay mineral surfaces. To achieve a strong bond between sodium polyacrylate and bentonite, the preparation process of modified soil can be divided into two processes: the grafting of triethoxyvinylsilane and the polymerization of the acrylic root monomer. The chemical reaction principle in the preparation process is described below.
-
(1) The graft reaction mechanism of bentonite is as follows:
(9)and $$ {CH}_2= CHSi{\left({OC}_2{H}_5\right)}_3+3{H}_2O\leftrightarrow {CH}_2= CHSi{(OH)}_3+3{C}_2{H}_5 OH $$
(10)where BNT is the bentonite (montmorillonite) group.
$$ {CH}_2= CHSi{\left({OC}_2{H}_5\right)}_3+3{H}_2O\leftrightarrow {CH}_2= CHSi{(OH)}_3+3{C}_2{H}_5 OH $$
(10)where BNT is the bentonite (montmorillonite) group. $$ {CH}_2= CHSi{\left({OC}_2{H}_5\right)}_3+ BNT{(OH)}_3\leftrightarrow BNT{(O)}_3 CHSi={CH}_2+3{H}_2\mathrm{O} $$
$$ {CH}_2= CHSi{\left({OC}_2{H}_5\right)}_3+ BNT{(OH)}_3\leftrightarrow BNT{(O)}_3 CHSi={CH}_2+3{H}_2\mathrm{O} $$
-
(2) The acidic environment, provided by acrylic acid, was conducive to silane hydrolysis and the dissolution of carbonate impurity minerals (calcite) in bentonite. This process unclogged the inner pores of clay particles. Finally, the excess acid was neutralized by NaOH to form sodium polyacrylate.
-
(3) N,N′-Methylenebisacrylamide was used as a crosslinking agent, which helps to form intersections in the polymerization process.
-
(4) K2S2O8 was used to trigger the reaction as an initiator.
The polymerization process occurs through a free radical reaction and involves chain initiation, chain growth, and end-of-chain termination:
-
(1) The acrylic monomers are separated into a linear chain due to the effect of the initiator (K2S2O8):
(11)
-
(2) The linear polyacrylic acid sodium then connects with the crosslinking agent (N,N′-methylenebisacrylamide), which results in the formation of intersections that begin to form a cross with each other:
(12)
-
(3) The branched chains from step (2) above start joining together and forming a three-dimensional network structure:
(13)
The length of the branched chain produced during this process is directly influenced by the concentration of acrylic acid, reaction temperature, and initiator concentration. On the other hand, the crosslinking density depends on the amount of N,N′-methylenebisacrylamide present. A high crosslinker density can reduce the composite’s swelling to a certain degree, which is an important consideration for specific applications.
XRD and CEC analysis
The XRD patterns (Fig. 2) demonstrate the complete removal of calcite in SAPAS-Bentonite, compared with raw bentonite, post-modification. This observation aligns with the formation of bubbles noted during the addition of acrylic acid in the preparation phase. The presence of calcite minerals indicates that the exchangeable cations in raw bentonite are primarily calcium, which can be replaced during the acidification modification process. Additionally, the primary montmorillonite peaks become broader and lower post-modification, suggesting alterations in the interlayer distance distribution. The acid introduced also dissolves minerals such as calcite, thereby reducing impurities and augmenting the specific surface area. A notable difference is an increase in CEC from 66.08 to 130.63 cmol kg–1 due to the modification. This increase is probably attributable to the acid activation process, which enhances the montmorillonite content and the activity of CEC. Furthermore, the introduction of sodium polyacrylate brings about an abundance of negative charges, thereby enhancing the cation adsorption capacity in comparison with raw bentonite.

Figure 2. XRD patterns of raw bentonite and SAPAS-Bentonite.
Thermogravimetric analysis
Figure 3 presents the thermogravimetric loss curves post-drying at 105°C, reflecting alterations in thermodynamic stability, strongly adsorbed water, and structural water following modification. Raw bentonite displays a 0.8% weight loss peak at 163°C, corresponding to the evaporation of interlayer strongly adsorbed water, where heat absorption occurs upon the breaking of hydrogen bonds (Lee and Char, Reference Lee and Char2002). However, SAPAS-Bentonite lacks this peak, suggesting a difference in its water adsorption and binding compared with raw bentonite. Owing to polymer interactions, SAPAS-Bentonite might exhibit poor water adsorption or non-uniform binding. Raw bentonite experienced a weight gain at 362°C due to aluminum oxidation, while SAPAS-Bentonite did not, probably because sodium polyacrylate supplied the necessary oxygen. Hydroxyl (structural water) weight loss occurred at 380–680°C for raw bentonite and 285–680°C for SAPAS-Bentonite, corresponding to the dehydroxylation of the bentonite sheet and the oxidation/volatilization of sodium polyacrylate. Despite the additional mass loss from organic carbon gasification, the final weight loss was 10.1% for SAPAS-Bentonite, which is less than that of raw bentonite (17.4%), indicating a significant improvement in thermal stability following modification.

Figure 3. The TGA/TG curves of raw bentonite and SAPAS-Bentonite.
Microstructures (SEM) analysis
The microstructure of raw bentonite, as observed under the scanning electron microscope, exhibited a tightly packed layered structure (Fig. 4a); sodium polyacrylate displayed a massive structure (Fig. 4b), while SAPAS-Bentonite contained a polyacrylic cementing the original layered structure (Fig. 4c). This observation suggests that the polymer in SAPAS-Bentonite is still small, and the polymer coating of minerals occurs at a much smaller nanoscale than depicted in the figure. The sharper sheets in SAPAS-Bentonite, compared with raw bentonite, may also be a result of the drying shrinkage of the sodium polyacrylate intercalated within the montmorillonite layers.

Figure 4. The SEM images of the material; (a) raw bentonite; (b) sodium polyacrylate; (c) SAPAS-Bentonite.
Adsorption and desorption results
Effect of contact time
Before and after modification, the lead ion adsorption-desorption kinetics of the bentonites exhibited similarities, with differences only in capacity and efficiency (Fig. 5a). In adsorption tests, both bentonites demonstrated rapid initial growth to equilibrium with stable plateaus, as the availability of adsorbable sites was reduced significantly. SAPAS-Bentonite reached adsorption equilibrium much faster, at 121 min, compared with 411 min for raw bentonite. This acceleration indicates enhanced lead diffusion to internal adsorption sites, probably due to calcite dissolution, montmorillonite activation, and sodium polyacrylate pore expansion during modification. The greater adsorption percentage of SAPAS-Bentonite also reflects the contribution of sodium polyacrylate (Chen et al., Reference Chen, Yu, Dong, Cai, Liao, Zeng and Ye2022). Similarly, SAPAS-Bentonite reached desorption equilibrium faster, at 62 min, compared with 360 min for raw bentonite. Both bentonites exhibited relatively low desorption, indicating stable adsorption. Given that SAPAS-Bentonite initially adsorbed more lead, the amount desorbed was also greater than that for raw bentonite.

Figure 5. Effect of contact time on adsorption and desorption of lead ions on the raw bentonite and SAPAS-Bentonite: (a) the adsorption and desorption curve; (b) pseudo-first order kinetic model; (c) second-order kinetic model (c i=1000 mg L–1, pH=4, T=25°C).
Pseudo-first order (Fig. 5b) and pseudo-second order (Fig. 5c) equations were employed to fit the adsorption kinetics. Table 4 presents the linear correlation coefficients, pseudo-first order rate constant (k1), pseudo-second order rate constant (k2), and equilibrium capacity values. A comparison of the R 2 values indicates that the adsorption kinetics of all bentonites better fit the pseudo-second order model, whether in adsorption or desorption. This suggests that the adsorption or desorption rates depend mainly on the lead ion concentration and are proportional to its square. After all, adsorption and desorption are a pair of positive and negative reactions, and their relative rates determine the direction of the adsorption reaction; the chemical mechanism remains the same. The faster adsorption rate of SAPAS-Bentonite can be attributed to differences in lead ion diffusion between the soil particles, facilitated by the water absorption and swelling of the polymer, which expands the diffusion channels. This diffusion control also applies to the desorption process.
Table 4. Values of parameters of pseudo-first order and pseudo-second order models for the adsorption of lead ions on raw bentonite and SAPAS-Bentonite

Effect of temperature
In the adsorption reaction, the transformation of the adsorbate from a free state to a bound state is generally considered a process of entropy reduction. The spontaneity of the adsorption reaction also signifies the exothermic nature of the reaction process. However, for bentonites dominated by cation exchange adsorption (including SAPAS-Bentonite), the adsorption of lead ions is accompanied by the desorption of adsorbable exchange ions (such as sodium or calcium ions) at the original adsorption site. The entropy change in this process is not necessarily reduced and may even increase at times. Therefore, some adsorption processes involving bentonite can also be endothermic. Indeed, the adsorption thermodynamics of bentonite currently hold two main viewpoints: endothermic (Donat et al., Reference Donat, Akdogan, Erdem and Cetisli2005; Karapinar and Donat, Reference Karapinar and Donat2009) and exothermic (Xu, Reference Xu2008; Taha et al., Reference Taha, Shreadah, Ahmed and Heiba2016). Regardless of whether it is endothermic or exothermic, the value of the enthalpy change is small, indicating that the adsorption of bentonite is primarily a physical process. This suggests that the effect of temperature aligns consistently with the experimental phenomena observed in the initial months. This consistency underscores the reliability of the experimental results and the robustness of the physical processes involved in bentonite adsorption.
The effect of temperature on the adsorption and desorption of raw bentonite and SAPAS-Bentonite reveals that with an increase in temperature, the adsorption percentage of lead ions by raw bentonite and SAPAS-Bentonite decreases slightly, while the desorption amount increases (Fig. 6). This indicates that the adsorption of lead by bentonite is an exothermic process. In contrast to adsorption, desorption is an endothermic process, wherein the influence of temperature on lead ions in desorption is lower than that in adsorption. When the temperature rises from 10 to 50°C, the desorption rate of adsorbed lead in the modified bentonite is 1.23%, which is larger than that of the raw bentonite at 0.6%. This demonstrates that the modified bentonite possesses good adsorption stability and also reflects that the adsorbed ion of polyacrylate is more easily desorbed than that of bentonite.

Figure 6. Effect of temperature on adsorption and desorption of lead ions on the raw bentonite and SAPAS-Bentonite (c i=1000 mg L–1, pH=4, t=720 min).
Effect of initial pH
The results of the adsorption and desorption of lead ions on raw bentonite and SAPAS-Bentonite (Fig. 7) demonstrate that the adsorption and desorption percentage curves of the two bentonites are similar. The adsorption capacity of SAPAS-Bentonite was found to be much greater than that of raw bentonite, particularly at pH>2.5. The decrease in pH of the adsorption system has a noticeable inhibitory effect on the adsorption performance of both types of bentonites, and it is more pronounced in SAPAS-Bentonite. The desorption results reveal that the lead adsorbed by bentonite is stable and the desorption amount is very small at solution pH>3. The decrease in pH results in the inhibition of lead adsorption and the promotion of desorption in bentonite, which is related to the competitive adsorption of H+. In the system, H+, as the competitive cation of lead ions, is bound to reduce the adsorption of lead ions when the total amount of exchangeable cation is fixed.

Figure 7. Effect of pH on adsorption and desorption of lead ions on the raw bentonite and SAPAS-Bentonite (c i=1000 mg L–1, t=720 min, T=25°C).
The competitive adsorption capacity of ions in bentonite depends mainly on the concentration, valence state, and ionic radius of ions (Chen et al., Reference Chen, Zhu, Wu, Wang, Yang, Ye and Guo2012). When pH>3, the concentration of hydrogen ions is small enough not to constitute significant adsorption competition because the pH is the negative logarithm of the concentration of hydrogen ions. In addition, after bentonite adsorbs enough hydrogen ions, the originally negatively charged mineral surface will be positively charged, which further inhibits the exchange adsorption of positively charged lead ions. The carboxylic acid group (-COO–) in SAPAS-Bentonite is a weak acid group, making it prone to binding hydrogen ions and rapidly reducing the negative charge on SAPAS-Bentonite. As a result, SAPAS-Bentonite is more sensitive to pH than raw bentonite.
Effect of initial concentrations of lead ions and isotherms models
The isothermal adsorption result serves as a key characterization of the pore properties of the adsorbent and the binding mode of the adsorbate. The maximum adsorption capacity of SAPAS-Bentonite for lead ions surpasses that of raw bentonite, and significant differences exist in the response of the two bentonites to the concentration of lead ions (Fig. 8). In terms of the adsorption curve, raw bentonite almost completely adsorbed lead ions at low concentrations (c i<600 mg L–1), while SAPAS-Bentonite could not. This phenomenon suggests that minerals such as montmorillonite and heulandite phases in SAPAS-Bentonite are indeed protected by sodium polyacrylate. The adsorption characteristics displayed at low concentrations indicate that the polymer is acting while the minerals in the soil particles do not seem to be exposed to lead ions because SAPAS-Bentonite actually possess full adsorption potential (Fig. 8a). This structural feature also causes the adsorption potential of clay minerals to be released and the adsorption rate to rise rapidly when the initial lead concentration increases and the sodium polyacrylate protection fails gradually. The failure of mineral protection in SAPAS-Bentonite is related to the increase in initial lead concentration and the gradual shrinkage of expanded sodium polyacrylate, leading to the opening of pores. SAPAS-Bentonite combines the adsorption properties of sodium polyacrylate and bentonite (Chen et al., Reference Chen, Yu, Dong, Cai, Liao, Zeng and Ye2022), and successively works to display an S-shaped isothermal adsorption curve (Fig. 8b). The first half of the adsorption, led by sodium acrylate, is a typical capillary coacervated adsorption due to the variable structure of the sodium polyacrylate (Chen et al., Reference Chen, Liao, Yu and Yu2020). The second half is similar to the raw material bentonite, exhibiting a single-layer adsorption characteristic.

Figure 8. Isothermal adsorption/desorption equilibrium results of the raw bentonite and SAPAS-Bentonite. (a) Percentage of lead ions adsorbed; (b) the amount of lead ions adsorbed; (c) Freundlich isotherm; (d) Langmuir isotherm (t=720 min, pH=4, T=25°C).
The characteristics of the desorption and adsorption curves of the two bentonites for lead are highly symmetric, as mentioned above. The comparison of adsorption capacity and desorption capacity at the same equilibrium concentration shows that the adsorption capacity is greater than the desorption capacity. This implies that some of the remaining lead ions cannot be completely desorbed. This phenomenon is probably caused by the pore structure of bentonite. In particular, the packaging protection of sodium polyacrylate prevents the lead ions adsorbed inside SAPAS-Bentonite particles from desorption, resulting in a higher retention rate of SAPAS-Bentonite than that of raw material bentonite.
The Langmuir and Freundlich models were employed to fit the adsorption and desorption data as a function of the linear form of the model (see Eqns (4) and (6)). The comparison of the fitting results of the two models (Table 5) reveals that the Langmuir isotherm model is more suitable for experimental data than the Freundlich model. SAPAS-Bentonite has two effective adsorption components, sodium polyacrylate and bentonite, and sodium polyacrylate has a ‘protective’ effect on bentonite at low concentrations (c i<1000 mg L–1). It can be considered as a two-stage adsorption, as described above. When the adsorption of these two phases reaches saturation adsorption capacity, the Langmuir isotherm model fits well for SAPAS-Bentonite. The maximum adsorption capacity of SAPAS-Bentonite was 165.73 mg g–1, which was much higher than that of the raw bentonite (112.36 mg g–1) according to the Langmuir isotherm model. It indicates that the modification process can significantly improve the adsorption capacity of bentonite to heavy metal ions. The maximum adsorption capacity calculated by desorption is greater than that calculated by adsorption. The retention of raw bentonite is ~16.17 mg g–1 (14% adsorption capacity), and that of SAPAS-Bentonite is 13.48 mg g–1 (8.4% adsorption capacity). By comparing the maximum adsorption capacity of lead ions with other adsorbents in literature, it can be found that the modification method and SAPAS-Bentonite in this study have excellent adsorption capacity of lead ions (Table 6).
Table 5. The parameters for Langmuir and Freundlich isotherms

Table 6. The maximum adsorption capacity of lead ions by various adsorbents in literature

Conclusions
Based on the discussion of the results, the following conclusions can be drawn:
-
(a) Triethoxyvinylsilane served as a grafting agent, facilitating the connection between montmorillonite and sodium polyacrylate. This interaction led to the formation of a stable chemical bond between the polymer and the clay mineral. The modified bentonite retained its layered structure but acquired a protective film of sodium polyacrylate. This outer layer of polymer acted as a ‘protective’ shield for the inner bentonite particles, playing a crucial role in fortifying the clay minerals.
-
(b) The adsorption and desorption of lead ions by raw bentonite or SAPAS-Bentonite are primarily affected by pH and initial concentration, with the effect of temperature being negligible. Under the same conditions, the trend of adsorption and desorption is similar, but the adsorption equilibrium is not consistent. Lead ions adsorbed by bentonite and SAPAS-Bentonite are not easily desorbed. Desorption capabilities of raw bentonite and SAPAS-Bentonite are low in conventional natural water environments with pH>3. In addition, ~14% of heavy metals adsorbed by bentonite are retained and challenging to desorb, while that of polycarboxylate is reversible. The isothermal adsorption pattern of raw bentonite is close to the Langmuir model, while SAPAS-Bentonite presents an S-shaped composite isothermal adsorption curve.
-
(c) Carboxylic groups exhibit distinct ion adsorption features compared with ion exchange in bentonite, effectively increasing its heavy metal adsorption capacity, with a greater priority than that of bentonite. The presence of polycarboxylate reduces the initial adsorption of heavy metals by bentonite until polycarboxylate saturation arises. The incorporation of 30% acrylic acid (relative to the quantity of raw bentonite) enhances the adsorption capacity for heavy metal lead by 47.5%, as indicated by the Langmuir isotherm model.
In summary, the adsorption and desorption characteristics of bentonite and SAPAS-Bentonite as adsorbents for lead ions were studied thoroughly here, providing a reliable evaluation and reference for the use and reuse of the two clays in heavy metals adsorption. In particular, the modification technology of polycarboxylate-grafted bentonite was proposed, effectively improving the adsorption capacity of heavy metals, and revealing the modification mechanism and special characteristics of ion adsorption. The SAPAS-Bentonite developed shows a broad application prospect in the treatment of heavy metal-contaminated soil and water.
Author contribution
Chuang Yu: Methodology; Writing - Review & Editing; Funding acquisition; Project administration; Supervision; Zhi-lei Zeng: Investigation; Visualization; Writing - Original Draft; Xiaoqing Cai: Resources; Formal analysis; Zhi-hao Chen: Formal analysis; Rao-ping Liao: Conceptualization; Investigation; Data Curation; Writing - Review & Editing.
Acknowledgements
none.
Financial support
This work was supported by the National Natural Science Foundation of China (grant number: 52178352), Zhejiang Provincial Natural Science Foundation (grant number: Ltgs24e080004), and the Plan Project of Science and Technology of Wenzhou (grant number: ZS2021001).
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.




















