Article contents
Adhered Zeolite Preparation on and Within a Muscovite Mica by Hydrothermal Growth
Published online by Cambridge University Press: 01 January 2024
Abstract
Zeolites and other open framework materials provide a powerful tool for remediation and solidification of a range of cationic wastes (e.g.NH4+${\rm{NH}}_4^ + $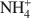 , Pb2+) due to the combined properties of large surface area and cation exchange capacity. However, practical barriers exist to the continued expansion of their use, including handling issues related to the fine particle size, and continued ion exchange following waste adsorption. This study examines the synthesis and characterization of zeolites adhered to a muscovite mica wafer, in order to assess if practical benefit can be derived from the preparation of layered composite materials. The paper demonstrates that increased metal adsorption, as demonstrated by surface chemical composition, can be induced in regions by growth of zeolite on and within the lamellar structure of the matrix. X-ray diffraction studies suggest that a site-specific crystallization mechanism controls the zeolite type and extent of growth, thereby reducing control over the zeolites prepared. However, although increased adsorption has been introduced to the mica, the amount of zeolite added is small (<50 mg per gram of muscovite), and thus any adsorption is very limited.
, Pb2+) due to the combined properties of large surface area and cation exchange capacity. However, practical barriers exist to the continued expansion of their use, including handling issues related to the fine particle size, and continued ion exchange following waste adsorption. This study examines the synthesis and characterization of zeolites adhered to a muscovite mica wafer, in order to assess if practical benefit can be derived from the preparation of layered composite materials. The paper demonstrates that increased metal adsorption, as demonstrated by surface chemical composition, can be induced in regions by growth of zeolite on and within the lamellar structure of the matrix. X-ray diffraction studies suggest that a site-specific crystallization mechanism controls the zeolite type and extent of growth, thereby reducing control over the zeolites prepared. However, although increased adsorption has been introduced to the mica, the amount of zeolite added is small (<50 mg per gram of muscovite), and thus any adsorption is very limited.
- Type
- Research Article
- Information
- Copyright
- Copyright © 2006, The Clay Minerals Society
References
- 4
- Cited by


