Published online by Cambridge University Press: 31 January 2022
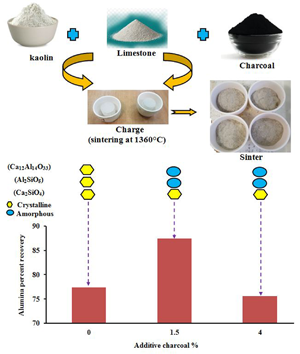
The present work aims to increase the alumina percentage recovery (APR) extracted from kaolin via the addition of 0.5–4.0 wt.% charcoal as a thermochemical fluxing agent in the lime-sintering process at 1260–1360°C. The transformation, microstructural and microtextural changes and self-disintegration performance were characterized using thermogravimetric analysis and differential scanning calorimetry, X-ray diffraction/X-ray fluorescence, scanning electron microscopy coupled with energy-dispersive spectroscopy and laser diffraction particle-size distribution analysis. The optimum enhancement of APR, from 77.7% to 87.40%, was obtained by sintering at 1360°C with the addition of 1.5% charcoal. With further increase of the charcoal content to 4%, the APR reduced to 75.6%. Combustion of ≤1.5% charcoal provided additional heat that amorphized the crystalline calcium aluminate into highly leachable amorphous phases with improved self-disintegration efficiency. Sintering at temperatures of >1360°C or with charcoal contents >4% led to mullite crystallization and decreased alumina leachability, thereby reducing the APR. Charcoal is a cost-effective and energy-efficient activator to increase the APR extracted from kaolin.
Editor: George E. Christidis