Article contents
Topotactic route to synthesis of novel hydroxylated phases: I. Trioctahedral Micas
Published online by Cambridge University Press: 09 July 2018
Abstract
K-depleted phlogopite mica was used as a topotactic precursor and treated with alkali (Li+, K+, 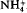 , Rb+, Cs+), alkaline-earth (Mg2+, Ca2+, Sr2+, Ba2+) and trivalent (Al3+) cations under hydrothermal conditions of 200°C and 30 MPa pressure. K-, NH4-, Rb- and Cs-aluminosilicate micas were synthesised at 200°C in one day. The synthesis of Cs-aluminosilicate mica, with potential applications in the management of nuclear wastes, has been achieved for the first time by this approach. Ion exchange by Li+, Na+ and alkaline-earth cations under hydrothermal conditions did not produce anhydrous mica phases but resulted in hydrous phases with one or two layers of water molecules between the clay layers. The formation of hydrous phases may be attributed to the high hydration energy of the above cations compared to K+,
, Rb+, Cs+), alkaline-earth (Mg2+, Ca2+, Sr2+, Ba2+) and trivalent (Al3+) cations under hydrothermal conditions of 200°C and 30 MPa pressure. K-, NH4-, Rb- and Cs-aluminosilicate micas were synthesised at 200°C in one day. The synthesis of Cs-aluminosilicate mica, with potential applications in the management of nuclear wastes, has been achieved for the first time by this approach. Ion exchange by Li+, Na+ and alkaline-earth cations under hydrothermal conditions did not produce anhydrous mica phases but resulted in hydrous phases with one or two layers of water molecules between the clay layers. The formation of hydrous phases may be attributed to the high hydration energy of the above cations compared to K+, 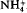 , RB+ and Cs+. Ion exchange with Al3+ produced a chlorite-like phase because of the hydrolysis of Al3+ under these hydrothermal conditions. These studies are of relevance in the immobilization of wastes where hazardous ions can be fixed in highly stable insoluble phases like mica or chlorite.
, RB+ and Cs+. Ion exchange with Al3+ produced a chlorite-like phase because of the hydrolysis of Al3+ under these hydrothermal conditions. These studies are of relevance in the immobilization of wastes where hazardous ions can be fixed in highly stable insoluble phases like mica or chlorite.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1986
References
- 9
- Cited by


