Advances in surgery and medical care have increased survival rates of children with univentricular heart malformations, and most survive into adulthood. Reference Schwartz, Mccracken, Petit and Sachdeva1 After a staged palliative surgery of the single ventricle, which results in a Fontan circulation, the burden of disease is complex and might include arrhythmias, liver fibrosis, and thrombosis. Reference Atz, Zak and Mahony2 From early childhood, these individuals experience challenges, regarding growth failure, low energy intake, and diminished exercise capacity. Reference Mccrindle, Williams and Mital3–Reference Hehir, Cooper, Walters and Ghanayem5 In infants with CHD that requires repair, a full catch-up growth is often achieved at 2 years of age; however, children with a univentricular heart disorder remain impaired in height throughout early childhood. Reference Daymont, Neal, Prosnitz and Cohen6,Reference Burch, Ravishankar and Newburger7 Recent studies on children with Fontan circulation have reported an increasing body mass index and low physical capacity, Reference Banks, Rosenthal and Manlhiot8,Reference Chung, Hong, Patterson, Petit and Ham9 but others have found reduced body mass index z scores in adolescents with single-ventricle physiology. Reference Aguilar, Raff, Tancredi and Griffin10 Although the consequences of different body compositions are described in the literature, studies on fat mass in the Fontan population are scarce. Reference Hansson, Lind, Ohlund, Wiklund and Rydberg11 One study showed that, amongst adolescents with Fontan circulation, greater leg muscle mass (lean mass) was associated with increases in cardiac output and systemic blood flow during exercise. Reference Avitabile, Goldberg, Leonard and Wei12 Another study showed that adolescents with vitamin D deficiency had reduced leg lean mass. Reference Avitabile, Leonard and Zemel13 Low levels of serum 25-hydroxyvitamin D appear to be equally common in the Fontan population as it is amongst healthy children. Reference Holler, Hannes and Germund14
Vitamin D is essential for calcium and skeletal homeostasis, and it is important in other tissues, including skeletal muscle. Reference Girgis, Clifton-Bligh, Hamrick, Holick and Gunton15,Reference Montenegro, Cruzat, Carlessi and Newsholme16 Vitamin D is stored in adipose tissue and requires 25-hydroxylation in the liver to become biologically active. In Fontan circulation, the liver is affected by continuous venous congestion, which can cause later hepatic complications, such as fibrosis. Reference Tellez, Rodriguez-Santiago and Albillos17 Vitamin D deficiency has been found in children and adolescents with hepatic diseases, Reference Zhu, Wang and Luo18 and studies have suggested that vitamin D-based treatments had a promising therapeutic potential for protecting against hepatic disease progression. Reference Elangovan, Chahal and Gunton19 Leg pain, similar to growth pain, is a common experience in the young Fontan population; however, no previous studies have addressed this symptom. In other studies, vitamin D supplementation has been suggested to reduce skeletal muscle pain. Reference Gendelman, Itzhaki, Makarov, Bennun and Amital20,Reference Wu, Malihi, Stewart, Lawes and Scragg21 Considering these findings, we reasoned that there might be an association between the Fontan circulation, vitamin D status, liver status, leg pain, and leg lean mass. The present study aimed to explore associations between vitamin D intake/serum 25-hydroxyvitamin D levels, liver biomarkers, leg pain, and body composition in young Fontan patients.
Materials and methods
Study populations
Study patients with Fontan circulation were recruited from Pediatric Cardiology Outpatient Clinics in the northern part of Sweden and in the region of Stockholm from September, 2017 to November, 2018. The study was conducted at the University Hospital in Umeå and at Karolinska University Hospital, Huddinge in Stockholm. Study eligibility criteria for all children included age 6–18 years, and no co-morbidity such as motor/cognitive difficulties that would affect the assessments of the dual-energy X-ray absorptiometry examination. To perform body composition comparisons, we recruited healthy, sex-, and age-matched children as a control group from the municipality of Umeå, by e-mail or telephone. Recruitments were performed by research nurses and other employees from the Pediatric Cardiology Outpatient Clinic in Umeå. The controls were screened prior to study enrolment to ensure that they did not have any previous cardiac medical history. Legal guardians of the children provided written informed consent.
Assessment of vitamin D intake and biochemical analyses in the Fontan patients
To relate levels of serum 25-hydroxyvitamin D with dietary intake of vitamin D, parents of the Fontan patients were asked to complete a validated food frequency questionnaire of food items and supplements known to be important sources of vitamin D. Reference Soderberg, Lind and Karlsland Akeson22 These foods included dairy products, eggs, and different types of fish. Food frequency was queried as the number of servings per day, week, or month. Portion sizes were assessed by the research dietician (LH). The Swedish Food Database 23 was used to estimate vitamin D intake (µg/day) from food. In the same questionnaire, patients were asked about visits to sunnier destinations and if they ever had experienced leg pain. Data on physical activity were also collected from a questionnaire regarding free time sports activity, participation in school sports, and estimated time of physical activity per day or week. Skin type was classified with the Fitzpatrick Skin Phototype Classification. Reference Fitzpatrick24 Based on pigmentation, the children’s skin types were divided into type I–III (fair) or type IV (light brown). To assess serum 25-hydroxyvitamin D and liver status, venous blood samples were collected. We determined the levels of 25-hydroxyvitamin D (nmol/L), parathyroid hormone (ng/L), aspartate aminotransferase (µkat/L), alanine aminotransferase (µkat/L), gamma-glutamyltransferase (µkat/L), and total cholesterol (mmol). Local anaesthetic cream (EMLA; AstraZeneca) was applied before blood sampling. Venous blood samples were taken in the fasting state and analysed at the Department of Clinical Chemistry, Umeå University Hospital in Umeå, and at Karolinska University Hospital in Stockholm, Sweden. We defined serum 25-hydroxyvitamin D levels >50 nmol/L as sufficient, <50 nmol/L as insufficient, and <25 nmol/l as severely deficient. Reference Ross, Manson and Abrams25,Reference Braegger, Campoy and Colomb26
Anthropometrics and body composition in Fontan patients and healthy controls
Body weight was measured to the nearest 0.1 kg with calibrated digital scales (Tanita scale, Tokyo, Japan), and height was measured to the nearest 0.1 cm with a wall-mounted stadiometer (Seca, Vogel & Halke, Hamburg, Germany) in both Umeå and Stockholm. Body composition was assessed with a dual-energy X-ray absorptiometry scanner (iDXA, Lunar iDXA, enCORE, Version 16, GE Health Care/Lunar Corp., Madison, WI, United States of America) in both hospitals, and it was administered according to the manufacturer’s protocol. To adjust and compare the two dual-energy X-ray absorptiometry devices in the two hospitals, one person was scanned in both devices. Three research nurses performed all scans according to standard clinical procedures. Whole-body dual-energy X-ray absorptiometry scans included bone mineral density, fat mass, lean mass, and bone mineral content for the evaluation of different tissue components. Lean mass was calculated by summing the estimated lean tissue mass and bone mineral content. Lean mass indexes and fat mass indexes were calculated by dividing the calculated mass (kg) by height squared (kg/m2). Appendicular (arms and legs) lean mass index and fat mass index were also calculated.
Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY, United States of America). Continuous data were expressed as the mean and standard deviation, and categorical data are expressed as the number and percentage. Multiple linear regressions were conducted to analyse the associations between vitamin D levels and biochemical tests, lean mass, fat mass, and leg pain in the Fontan group. Regression models were adjusted for age, sex, and season of blood collection. Seasons were divided into autumn and spring. Differences in biochemical analyses between different age and sex groups were evaluated with the independent sample t-test. Reference values for the biochemical analyses were based on the previously published 2.5 and 97.5 percentiles of data from healthy individuals. Reference Aldrimer, Ridefelt and Rödöö27 Data were converted to z scores by assuming that the reference values were based on normally distributed data, and the mean value was approximated by the average of the high and low percentile values. The reference values were constant for different age ranges; therefore, we used linear interpolation to calculate values in the gaps between age groups. z scores were calculated by subtracting the mean at the corresponding age, then dividing by the standard deviation for the same age.
Differences in mean body composition values between children with Fontan circulation and controls were assessed with the paired sample t-test. The chi-square test was used to assess differences in physical activity levels. Data were divided into two subgroups that represented children aged 6–12 years and aged 13–18 years. The cut point for stratification by age was chosen based on the substantial physiological development of muscle function during puberty. Reference Dotan, Mitchell, Cohen, Klentrou, Gabriel and Falk28 The differences were most pronounced in adolescents aged 13–18 years, and therefore only this group is reported in the present study. Stratification by sex was also conducted between the Fontan patients and controls. Significant differences were defined as p < 0.05.
Results
Study population
In total, 78 children with Fontan physiology were identified (Fig 1). Of these, 32 declined participation, due to lack of interest, discomfort with the examination and/or the dual-energy X-ray absorptiometry scan, or failure to reply to the invitation. Two children were excluded due to co-morbidity (Down syndrome). Thus, a total of 44 children with Fontan circulation were enrolled in this study. Characteristics of the Fontan patients are summarised in Table 1. No one in the Fontan population had an imaging evaluation of liver fibrosis. The 32 children with Fontan circulation that declined study participation were not different in age or sex compared to the included children. Of the 44 children with Fontan circulation included, 38 were available for dual-energy X-ray absorptiometry scans; therefore, 38 sex- and age-matched healthy controls were recruited for body composition comparisons.

Figure 1. Flowchart.
Table 1. Characteristics of 44 children and adolescents with Fontan circulation.

Values are the mean (standard deviation), unless otherwise indicated; TCPC = Total Cavo-Pulmonary Connection.
a Biochemical reference values for different ages and sex were converted to z scores by subtracting the mean value at the corresponding age and sex, divided by the SD for the same age.
Skin pigmentation; Fitzpatrick Scale, type I–III = fair skin, IV = brown skin.
** Cholesterol z score was significantly different from females at p < 0.001.
Biochemical analyses, vitamin D intake, and body composition in Fontan patients
Table 1 shows the biochemical analyses, seasonal blood sampling data, vitamin D intake, and skin types of the children with Fontan circulation. The mean vitamin D intake from foods was consistent with the recommended intake of 10 µg/day. 29 Only six (15%) patients with Fontan circulation had received vitamin D supplementation. The mean serum 25-hydroxyvitamin D level was 56 nmol/L, above the sufficient level (50 nmol/L), but 42% of the Fontan population had insufficient levels (<50 nmol/L). Only one patient with Fontan circulation had severe vitamin D deficiency (<25 nmol/L). No correlation between vitamin D intake and serum 25-hydroxyvitamin D was observed. Parathyroid hormone levels were available for 21 children, and all values were within the normal range. Of the 44 children with Fontan circulation, 34% experienced leg pain. Biochemical analyses of liver status as z score were different from normal reference values. The z scores for aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyltransferase were higher than the reference values, conversely, the total cholesterol z score was lower than the reference value. Moreover, amongst the patients with Fontan circulation, males had significantly higher total cholesterol z scores than females (Table 1). In the multiple linear regression model constructed to assess the associations between biochemical analyses of liver status and 25-hydroxyvitamin D, we found that gamma-glutamyltransferase and total cholesterol was not associated with serum 25-hydroxyvitamin D and total cholesterol were not associated with leg pain. Furthermore, no association was observed between serum 25-hydroxyvitamin D and leg pain, bone mineral density, the lean mass index, or the fat mass index.
Anthropometrics, physical activity, and body composition in Fontan patients and controls
The two dual-energy X-ray absorptiometry devices located in Stockholm and Umeå were validated, and no data adjustment was needed. The anthropometric data and the dual-energy X-ray absorptiometry results are shown in Table 2. The Fontan group was significantly shorter in height than the control group, but the groups were similar in weight, body mass index, and the body mass index z score adjusted for age. The children with Fontan circulation were significantly less active than controls, during free-time physical activity. No difference between groups was found in the bone mineral density z score. Compared to controls, the Fontan patients had lower lean mass index scores in both arms and legs, and significantly higher fat mass index scores in the arms, abdomen, and total body. When the groups were divided by age, (i.e., 6–12 and 13–18 years), we observed a more distinct difference in body composition. The younger Fontan group (6–12 years), had a significantly higher abdominal fat mass index than controls, but no difference was found in the fat mass index or lean mass index in other body parts or appendicular indexes (data not shown). The older Fontan group (13–18 years) had significantly lower lean mass index scores in the arms, legs, and total body, and significantly higher fat mass in the abdomen and total body compared to controls (Table 3). Amongst females, no differences in fat mass index scores were observed between the Fontan and control groups (data not shown). However, amongst males, the Fontan group had significantly higher fat mass index scores in the arms, legs, abdomen, and total body, compared to healthy controls.
Table 2. Body composition of 38 children and adolescents with Fontan circulation and 38 healthy controls from 6 to 18 years old.
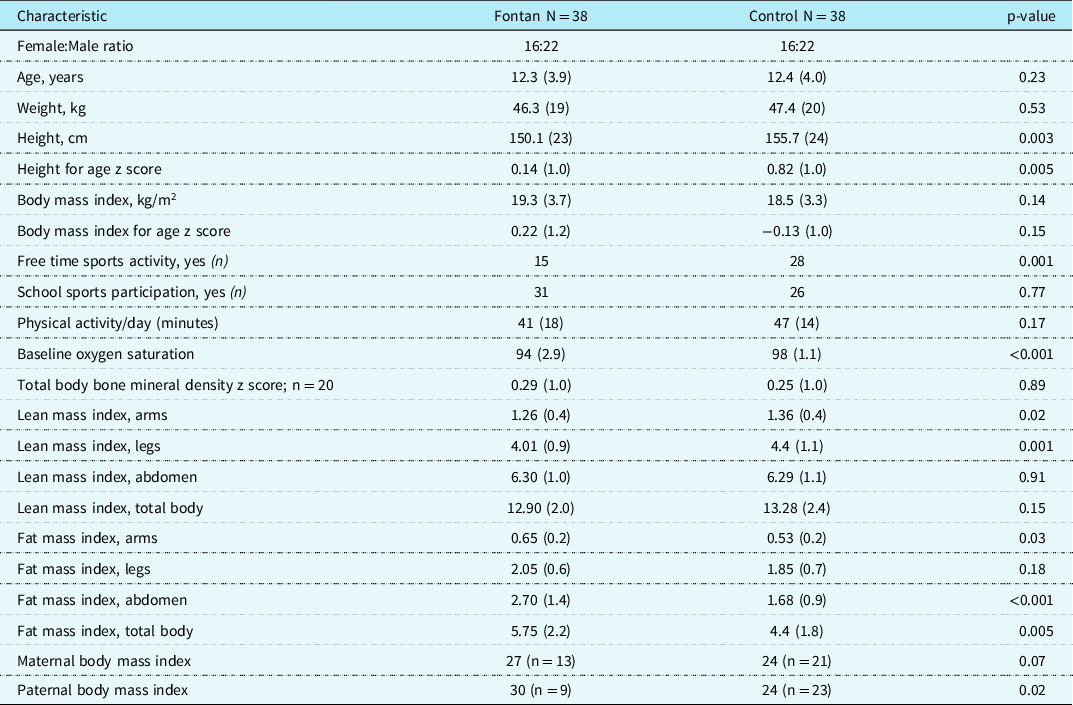
Values are the mean (standard deviation), unless otherwise indicated.
Group differences of body composition were analysed with the paired sample t-test. The Chi-square test was used to assess differences in physical activity levels. Height for age was converted to z score using the WHO Anthro+ software for personal computers, version 3.2.2, 2011 (Geneva, WHO, 2010).
Table 3. Body composition of 20 adolescents with Fontan circulation and 20 healthy controls from the age of 13 to 18 years.
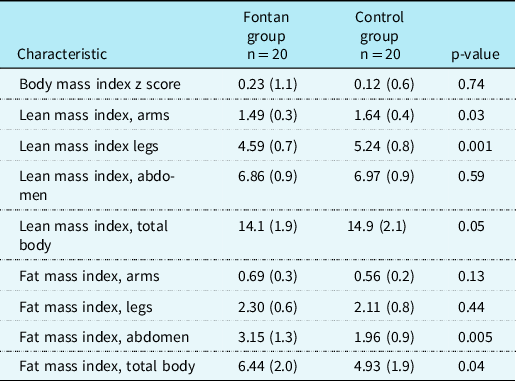
Values are the mean (standard deviation).
Group differences were analysed with the paired sample t-test.
Discussion
In this observational study, we demonstrated that the Fontan patients had a mean dietary vitamin D intake consistent with Nordic Nutrition recommendations. 29 The mean serum 25-hydroxyvitamin D was sufficient, and it was not associated with the lean mass index, fat mass index, leg pain, or biochemical analyses of liver status. However, we found that children and adolescents with Fontan circulation had significantly less lean mass in the arms and legs than healthy controls. Conversely, the Fontan patients had significantly more pronounced fat mass in the total body and in the arms and abdomen compared to healthy controls.
We found that, although the mean level of serum 25-hydroxyvitamin D was 56 nmol/L, 42% of children had levels below 50 nmol/L. However, severe vitamin D deficiency (<25 nmol/L) was uncommon. These findings are interesting, because pre-vitamin D is metabolised to 25-hydroxyvitamin D3 in the liver, and individuals with a univentricular heart experience hepatic changes secondary to the Fontan circulation, mainly due to the passive venous overload in the liver. Reference Goldberg, Surrey and Glatz30 This condition might disturb the hydroxylation of vitamin D. Thus, based on this association between vitamin D status and hepatic function indexes, we hypothesised that low serum 25-hydroxyvitamin D might have been related to poor liver function. Reference Petta, Camma and Scazzone31 However, in the present study, we found no significant link between the biochemical analyses related to liver status and serum 25-hydroxyvitamin D levels. Therefore, the potential relationship between hepatic function and serum 25-hydroxyvitamin D levels was not detected in our patients. Hepatic fibrosis is a well-known, long-term complication in patients with TCPC and the key to optimising the prognosis is early detection. Unfortunately, the current methods such as radiology or laboratory parameters cannot detect Fontan-associated liver disease at an early stage. Reference Komatsu, Inui and Kishiki32 In this study, the mean serum 25-hydroxyvitamin D levels were lower than the 75 nmol/L reported in two previous studies on young Fontan patients. Reference Avitabile, Leonard and Zemel13,Reference Sarafoglou, Petryk and Mishra33 However, our results were consistent with a recent study by Holler and co-workers, Reference Holler, Hannes and Germund14 who found that 72% of their Fontan group had insufficient serum 25-hydroxyvitamin D levels, and the mean value was 35 nmol/L. They found that insufficient and severely deficient vitamin D levels were associated with skin type. Fair skin is potentially more effective in producing vitamin D; thus, skin type I–III might be more beneficial for children with Fontan circulation in northern latitudes. Reference Ohlund, Lind, Hernell, Silfverdal and Karlsland Akeson34 In general, the Swedish population appears to have higher serum 25-hydroxyvitamin D levels than populations in other countries; Reference Hilger, Friedel and Herr35 however, this was not true in our study population of Swedish children and adolescents with Fontan circulation. In our study group, skin type could not explain the vitamin D deficiencies observed, although we only included four children (11%) with a darker skin type.
In this study, we also analysed the dietary vitamin D intake. The mean dietary vitamin D intake was in accordance with recommendations. This result might be explained by the general vitamin D fortification in milk and bread spreads and by the school meals (lunch and a small afternoon meal), which are free for all school children in Sweden and based on Nordic nutritional recommendations. The vitamin D intake from other food items, such as fish and other fortified dairy products, might have also contributed to the levels of serum 25-hydroxyvitamin D. Unfortunately, we found that the vitamin D intake, though in accordance with the recommended intake, did not have a beneficial effect on serum vitamin D levels in our Fontan patients. It should be stated that bone health is used as a marker for adequate vitamin D intake, and therefore the recommended levels for vitamin D intake might be insufficient in this particular group of individuals with a univentricular heart and liver changes secondary to the Fontan circulation.
This study was the first to evaluate vitamin D intake in young Fontan patients. Considering findings from the current and earlier studies, which showed deficient vitamin D status in Fontan patients, it might be important to regularly monitor vitamin D, and generously supplement these children when necessary. In the present study, only 15% of the Fontan patients received vitamin supplementation, although 42% had levels below the sufficient level of serum 25-hydroxyvitamin D.
In a previous systematic review and meta-analysis from Wu et al, muscle pain was associated with reduced vitamin D levels. Reference Wu, Malihi, Stewart, Lawes and Scragg21 In the present study, 34% of the Fontan patients experienced leg pain. This is a common symptom, based on clinical knowledge from paediatric cardiology clinics; however, it had not been studied previously. In our cohort, leg pain did not associate with vitamin D status. Moreover, previous studies showed that low serum 25-hydroxyvitamin D levels were associated with reduced lean mass. Reference Avitabile, Leonard and Zemel13 However, in the present study, we found no correlation between serum 25-hydroxyvitamin D and body composition in our Fontan patients.
Recent studies by Avitabile et al have suggested that the lean mass in individuals with Fontan circulation differed from the reference standards. Reference Avitabile, Goldberg, Leonard and Wei12,Reference Avitabile, Leonard and Zemel13 The authors found normal body mass index z scores, but below-normal lean mass index scores for the total body and the legs. Consistent with those findings, we found that our Fontan patients had body mass index z scores comparable to controls, but lower lean mass index scores for both the arms and legs. Moreover, in the Fontan group of patients 13–18 years old, we also found lower lean mass index scores compared to controls. These observations could be important, particularly in this patient group, because the lower limb muscle group pumps blood to the heart, and this activity is vital and key to delivering non-oxygenated blood to the heart. In a study by Cordina et al, high-resistance training in adults with Fontan circulation resulted in improvements in muscle mass and strength, and these improvements were associated with improvements in stroke volume, cardiac output, and exercise capacity. Reference Cordina, O’meagher and Karmali36
Fontan physiology has been associated with a low bone mineral density z score. Reference Avitabile, Goldberg and Zemel37–Reference D’ambrosio, Tran and Verrall39 This is a particular concern in young children with Fontan circulation, because early bone mass derangements could lead to osteoporosis later in life. In the present study, we found no significant difference in bone mineral density z scores between our Fontan patients and the matched control group, however, data were limited. Further, our Fontan patients spent significantly less free time in sports activities compared to their healthy peers. Physical activity has a beneficial effect on both bone density and lean mass; thus, individualised training programs have improved exercise capacity and quality of life in patients with Fontan circulation. Reference Hedlund, Lundell, Söderström and Sjöberg40 However, it should be emphasised that weight-bearing activities, specifically, are required to increase bone density. Reference Gómez-Bruton, Matute-Llorente, González-Agüero, Casajús and Vicente-Rodríguez41
In this study, we explored possible links between vitamin D status and body composition. Surprisingly, we found new interesting results regarding fat mass. Studies on fat mass in children with Fontan circulation are scarce. The focus has been on overweight status or high body mass index. Reference Chung, Hong, Patterson, Petit and Ham9,Reference Pasquali, Marino and Pudusseri42,Reference Welisch, Rauch, Seabrook, Filler and Norozi43 Those studies have shown that Fontan circulation was associated with a high risk of overweight status, but the risk was comparable to that of healthy children. In other words, the Fontan patients were not excluded from the obesity epidemic. Importantly, our study showed that compared to healthy controls, the Fontan patients had a significantly higher total body fat mass index, higher abdominal fat mass index scores, and higher arm fat mass index scores than controls, despite their comparable body mass index z scores and weights. We previously showed that 9-year-old children with diverse complex CHD, including Fontan circulation, had a high abdominal fat mass index, compared to healthy controls, despite their comparable body mass index z scores and lean mass indexes. Reference Hansson, Lind, Ohlund, Wiklund and Rydberg11 In this present study, a higher abdominal fat mass was also observed in children aged 6–12 years old and in children and adolescents aged 13–18 years old. That finding was consistent with findings from a recent study by Tran et al, who showed an elevated fat mass index in young adults with Fontan circulation. Reference Tran, D’ambrosio and Verrall44 These results might suggest that an elevated abdominal fat mass persists after adolescence in individuals with Fontan circulation.
Dual-energy X-ray absorptiometry scans have been shown to provide a valid measure of abdominal adiposity. Reference Hill, Laforgia, Coates, Buckley and Howe45 A previous study on visceral adipose tissue in children measured with dual-energy X-ray absorptiometry showed associations with cardio-metabolic risk factors, such as serum lipids, insulin resistance, and diastolic blood pressure. Reference Bosch, Dengel and Kelly46 Abdominal fat mass is a well-known, strong marker for increased risk of cardiovascular disease later in life, Reference Aune, Sen and Norat47 and adults with Fontan physiology share this risk with other populations. Reference Schwartz, Mccracken, Petit and Sachdeva1 In the present study, children with Fontan circulation had significantly lower cholesterol levels compared to the mean z score of reference cholesterol levels. Nonetheless, the males with Fontan circulation in this study had higher cholesterol levels than females with Fontan circulation. Moreover, males with Fontan circulation had higher abdominal fat mass index scores than control males, which might have indicated a higher risk of continued abdominal fat mass in adulthood in the male group. Therefore, higher total and abdominal fat mass indexes in children with Fontan circulation could be a marker for increased risk of metabolic disturbances later in life. We speculate that, in this population characterised by faltering growth in infancy, followed by accelerated weight gain without height gain, and high abdominal fat mass in adolescence, might have a high risk of later cardiovascular disease. Reference Singhal and Lucas48 Because Fontan circulation puts this group of patients at high risk of several complications, an additional burden of disease due to a higher fat mass status should be avoided.
This study had several limitations and strengths. The main limitations included the observational study design and the small sample size, although our group was larger than other studies on children with Fontan circulation. Nevertheless, a considerable number of children with Fontan circulation declined participation in our study. One explanation for this decline rate might be that this small group of interesting patients is frequently asked to participate in studies, and they might feel overextended. In the present study, the distributions of age and sex did not differ between patients and non-patients; nonetheless, due to the lack of data on body composition and vitamin D status in the non-patients, a selection bias could not be excluded. A recent study suggested that parts of the Fontan population might have a higher incidence of delayed puberty. Reference Menon, Al-Dulaimi and Mccrindle49 Unfortunately, we did not have access to documentation on the level of puberty in our study patients; thus, another limitation was that we could not determine the potential impact of puberty on our results. Arrhythmias and medication could possibly affect physical performance; unfortunately, data were not available. The study was also limited, because we could not compare the Fontan group to healthy controls, regarding nutritional intake, vitamin status, or leg pain. This comparison could have provided additional information about the Fontan patients. Another potential limitation was that the control and Fontan groups were not comparable in some aspects. For example, the paternal body mass indexes differed between groups. Also, the controls were recruited from a university city (Umeå), and the Fontan patients were recruited from all over the northern counties and Stockholm. However, the study results showed no differences between the Fontan patients and controls regarding body mass index, weight, or maternal body mass index. Therefore, we are inclined to assume that socio-economic differences could not explain the differences between groups observed in our study.
The strengths of this study included the fact that we accessed data on dietary vitamin D intake, the season of blood sampling, and the travel to sunny latitudes, which could be associated with serum 25-hydroxyvitamin D levels. This comprehensive data set had not been reported previously in a Fontan population. Additionally, the study population was recruited from a large geographical area of Sweden; therefore, the study results might be generalisable to a high proportion of Swedish children with Fontan circulation.
The results of this study indicated that there are opportunities for improving rehabilitation in this group of children and adolescents. However, this possibility should be confirmed in a larger cohort of individuals with Fontan circulation.
Conclusion
This study demonstrated that, although vitamin D intake was in accordance with recommendations, 42% of the Fontan patients had <50 nmol/L serum 25-hydroxyvitamin D. Moreover, we found no association between vitamin D status and body composition or biochemical analyses of liver status, in children with Fontan circulation. However, the Fontan patients had a lower lean mass and higher fat mass compared to healthy controls. A more pronounced abdominal fat mass in children and adolescents with Fontan circulation might be a marker for increased risk of metabolic disturbances later in life.
Acknowledgements
We would like to thank all the participating children and adolescents.
Financial support
Financial support was provided through the Swedish Heart-Lung Foundation (20160496).
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work complied with the Helsinki Declaration of 1975, as revised in 2008. This study was approved by the Regional Ethics Review Board, in Umeå, Sweden (2018–07–32M).







