What we know
-
Good growth in infants born with CHD is associated with improved surgical outcomes.
-
Growth failure in infants born with CHD is a persistent problem, even in those provided with adequate nutrition.
-
Aberrant growth during the first 2 years of life may be associated with poorer metabolic outcomes later in life.
What this study adds
-
There are considerable gaps in our knowledge relating to how metabolic maturation occurs in infants with CHD, specifically:
-
What factors are associated with metabolic instability and developmental delay.
-
Which metabolites are useful to monitor with regards to haemodynamic stability and growth outcomes, and how do these change over time in infants with CHD.
-
In the small number of cases studied, there is an accumulation of metabolites associated with the tricarboxylic acid cycle, with a potential downstream effect on the availability of ketone bodies necessary for the developing brain and growth.
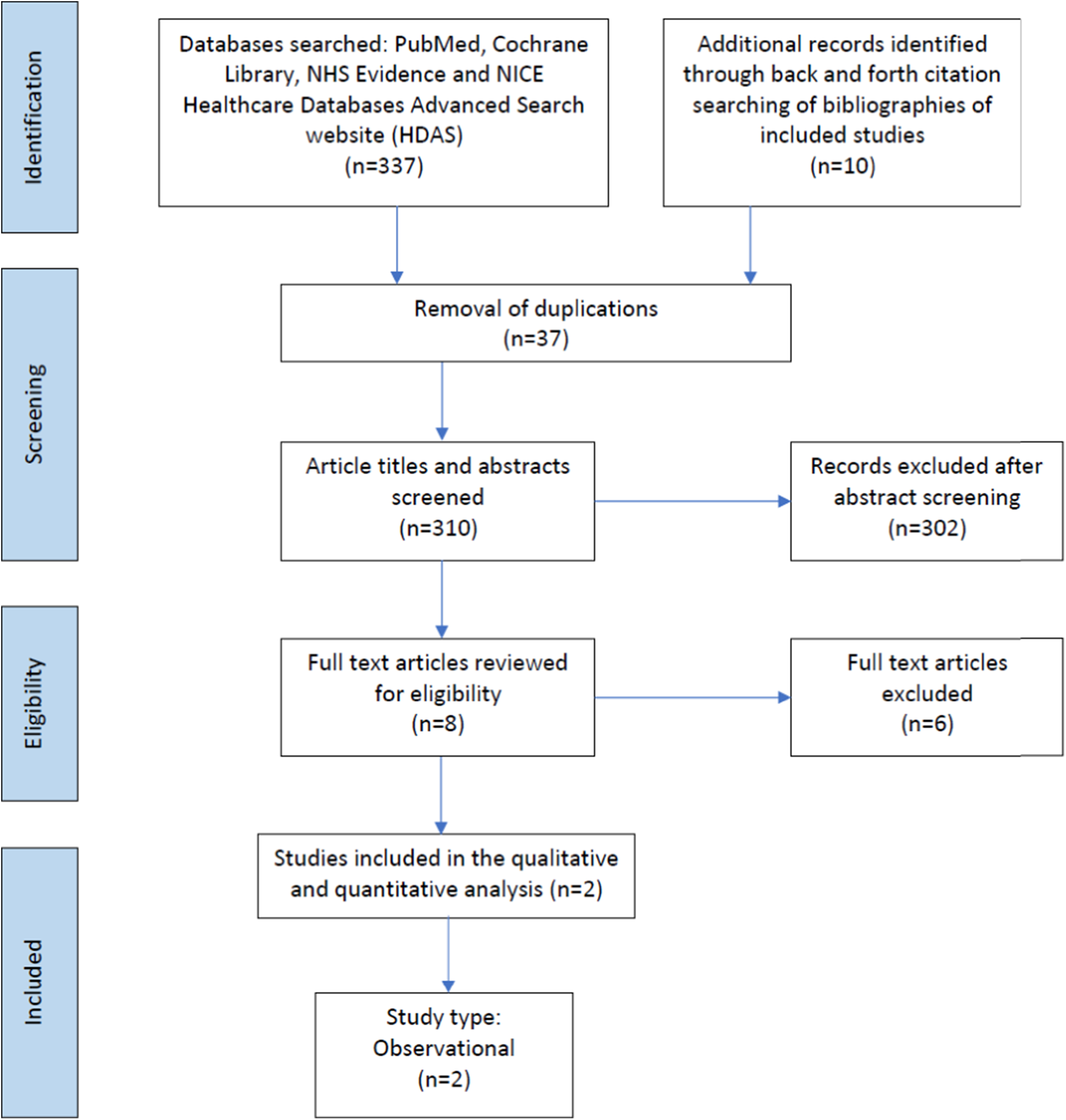
Figure 1. Prisma flow chart of studies included in the scoping review. Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for Scoping Reviews (PRISMA-ScR) checklist.
CHD represents one-third of all major congenital anomalies, with a reported prevalence of 9 per 1000 live births [95%CI:8.1–9.3]. Reference van der Linde, Konings and Slager1 During the past 50 years, there have been significant improvements in the medical and surgical management of CHD, with more children now reaching adulthood. Reference van der Linde, Konings and Slager1 Improved survival, especially of infants with complex CHD lesions, is associated with increased risk of neurodevelopmenal delay. Reference Wernovsky and Licht2 Brain maturation and altered cerebral blood flow have been described in infants with CHD in utero and following birth. Those infants requiring medical, surgical, or catheter-based procedures especially in the first few weeks of life may be impacted by inadequate cerebral blood flow, hypoxaemia, and cerebral embolism. Reference Marino, Lipkin and Newburger3 Other factors impacting neurodevelopment delay include mechanical support provided during surgery, including cardiopulmonary bypass and haemodilution, hypothermia, glucose management, poor cardiac output, and hypotension post-operatively. Reference Wernovsky and Licht2 Work is ongoing to identify modifiable risk factors to improve long-term neurodevelopmental outcomes. Reference Wernovsky and Licht2,Reference Marino, Lipkin and Newburger3
Infants with CHD represent a heterogenous group. Reference Diao, Chen and Wei4 Pre-operative malnutrition is a persistent problem with the prevalence of underweight for age reported as 27.4% (95% CI, 21.7–34.0), 24.4% (95%CI, 19.5–30.0) for stunting, and 24.8% (95% CI, 19.3–31.3) for wasting. Reference Diao, Chen and Wei4–Reference Toole, Toole, Kyle, Cabrera, Orellana and Coss-Bu9 Infants with CHD who have persistent malnutrition at the time of surgery have poorer post-operative clinical outcomes irrespective of their cardiac lesion or complexity of surgical intervention. Reference Eskedal, Hagemo and Seem10,Reference Mitting, Marino, Macrae, Shastri, Meyer and Pathan11 These include an increased risk of cardiac arrest, infection, Reference Ross, Latham and Joffe12 prolonged ICU stay, Reference Marino, Griksaitis and Pappachan7 total length of hospital stay, Reference Marino and Magee6,Reference Toole, Toole, Kyle, Cabrera, Orellana and Coss-Bu9 and increased risk of mortality at both 3 and 12 months of age. Even if catch up growth is achieved later in childhood, if this occurs after 2 years of age, it is associated with an increased risk of metabolic disease. Reference Aguilar, Raff, Tancredi and Griffin13,Reference Jackson, Fox, Cotto, Harrison, Tran and Keim14 In addition, poor growth and feeding difficulties amongst infants with CHD cause parents significant distress and worry. Reference Tregay, Brown, Crowe, Bull, Knowles and Wray15,Reference Marino, Johnson and Davies16
Even in children treated with a structured nutrition pathway, designed to provide intensive nutrition from birth to the time of surgical intervention, 20% remain underweight and 14% remain stunted at the time of surgery. Reference Marino, Johnson and Davies8 Giallouri et al. have previously shown that malnourished children in resource-constrained environments show time-dependent changes in metabolites derived from nutrient–gut microbial–host metabolic interactions, and muscle-associated molecules (energy- and choline-related metabolites). Reference Mayneris-Perxachs and Swann17,Reference Giallourou, Fardus-Reid and Panic18 Using this approach, age-specific reference curves for urinary metabolites associated with growth in malnourished children were obtained and phenome-for-age z-scores for each child (a measure of biochemical maturity) were calculated. This work suggested that nutritional interventions could be targeted using metabolic rather than chronological age. Reference Giallourou, Fardus-Reid and Panic18
Using advanced analytics, complex metabolic signatures (metabolomes) can be characterised and integrated with conventional anthropometric and biological data to identify novel nutritional phenotypes. This systems biology approach can increase the molecular and temporal resolution at which the biochemical status of these individual children can be studied. Such metabolic phenotypes can highlight the dynamic nutritional demands of the child, providing detailed information against which personalised nutritional support can be tailored. Reference Mayneris-Perxachs and Swann17 1H nuclear magnetic resonance (1H NMR) spectroscopy and mass spectrometry are high-resolution analytical chemistry techniques that can be used to comprehensively profile biofluid (urine, blood, saliva) and tissue metabolomes. Reference Giallourou, Fardus-Reid and Panic18 Such analyses, particularly in non-invasive samples such as urine, can provide a clinical tool to better understand the maturing metabolism of children with CHD, its relationship to growth, Reference Mayneris-Perxachs and Swann17,Reference Giallourou, Fardus-Reid and Panic18 response to nutrition interventions as well as identifying target for nutritional supplementation. This may provide an opportunity to refine nutritional recommendations and consider targeted supplementary interventions to ensure improved growth outcomes. Reference Giallourou, Fardus-Reid and Panic18–Reference Bourdon, Lelijveld and Thompson21
The aim of this scoping review is to review the evidence describing associations between urinary metabolomics, nutrition, and growth in infants with CHD and characterise metabolites that could be used to develop phenome-for-age z scores.
Methods
A scoping review was conducted to assess the evidence available relating to urinary metabolites and growth in infants with CHD. The scoping review framework facilitates the analysis of a broad range of evidence in an emerging field and the identification of gaps in knowledge to direct future research priorities. Reference Munn, Peters, Stern, Tufanaru, McArthur and Aromataris22 The Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Reference Tricco, Lillie and Zarin23 was used to report the evidence examined in this review.
Protocol development
The PRISMA-ScR checklist Reference Tricco, Lillie and Zarin23 was used to develop a protocol. Reference Marino, Valla, Beattie and Verbruggen24 The protocol described the research question, the information sources to be searched, a description of the full electronic search strategy, study inclusion and exclusion criteria, data extraction and charting, data collection, analysis, and critical appraisal to answer the research questions posed.
-
1. The research questions
-
a. Do infants with CHD have abnormal metabolic maturation?
-
b. Are there specific metabolic signatures associated with appropriate growth in infants with CHD?
-
-
2. Data Sources Searched
The databases searched were PubMed, the Cochrane Library, NHS Evidence, and the NICE Healthcare Databases Advanced Search website (HDAS) (https://hdas.nice.org.uk/). HDAS was used to allow searches within multiple databases, including, PsycInfo, Cumulative Index to Nursing and Allied Health Literature, and Medline.
-
3. The Search Strategy
A search strategy, using key words from articles relating to infants born with CHD (Supplementary File: Table 1), was devised with the assistance of a library information specialist. Search terms included infants or neonates; CHD; urinary metabolomics or urinary metabolites; growth or weight gain; metabolic maturation or metabolic maturity. Searches were adapted for the additional electronic databases. Forward and backward citation searching was completed until February 2022.
-
4. Study Selection
Studies were eligible for inclusion if they were published before February 2022 in the English language, based on human subjects, and used a qualitative or quantitative design to describe urinary markers of metabolic maturation or growth in infants with CHD. Opinion pieces, editorials, and congress abstracts were excluded as per the scoping methodology advocated by Aksey and O’Malley. Reference Arksey and O’Malley25 Article titles and abstracts were screened, duplicates deleted, and then full-text articles reviewed for eligibility (SP, LVM, JJA, CW). Where multiple articles described the same cohort of children, these were only counted once. Bibliographies of included studies were hand searched for additional studies which may fulfil the inclusion criteria. Exclusion criteria included preterm infants with patent ductus arteriosus.
Data extraction and charting
A two-stage process was completed as part of data extraction. A data extraction template (Microsoft 2010, Redmond, WA) was used to capture the study design, results, and conclusions, followed by a content analysis.
-
5. Collating, summarising, and reporting results
Content analysis was chosen for reporting subjects common to the data sets. Reference Arksey and O’Malley25,Reference Levac, Colquhoun and O’Brien26 Descriptive aspects about the population studied, methodology, outcomes, and any key findings were coded. Initial themes, identified from the key findings, were grouped into sub-categories, and then into overarching themes. The overarching themes and sub-categories from this process were used to develop a conceptual framework.
Results
Study characteristics
347 records were identified, of which 37 were duplicates. Following the removal of duplicate records, 310 abstracts and titles were screened for inclusion. The full texts of eight articles were reviewed for eligibility, of which two related to infants with CHD (Table 1). The studies included in the scoping review described urinary metabolites in 42 infants. Reference Simonato, Fochi and Vedovelli27,Reference Vedovelli, Cogo and Cainelli28 The studies identified infants with CHD who had poorer neurodevelopment outcomes post-surgery excreted greater amounts of certain metabolites pre-surgery. This included metabolites associated with energy metabolism (glucose, sucrose, succinate, 2-oxoglutarate, lactate), gut microbial metabolism (acetate, formate), creatine, and urea. Surgery was also found to disrupt gut microbial metabolism in infants with CHD altering the excretion of the gut microbial-host co-metabolite, phenylacetylglutamine, as well as the primary conjugated bile acid, glycocholic acid, and the indolic compounds, indole-3-aldehyde, and indoleacrylic acid. The greatest impact of surgery involving cardiopulmonary bypass was related to inflammation and modulation of the tryptophan-kynurenine pathway, with tryptophan, kynurenine, 3-OH-kynurenine, and kynurenic acid excretion altered post-surgery. Reference Simonato, Fochi and Vedovelli27,Reference Vedovelli, Cogo and Cainelli28
Table 1. Studies describing urinary metabolites in infants with CHD. Reference Simonato, Fochi and Vedovelli27,Reference Vedovelli, Cogo and Cainelli28
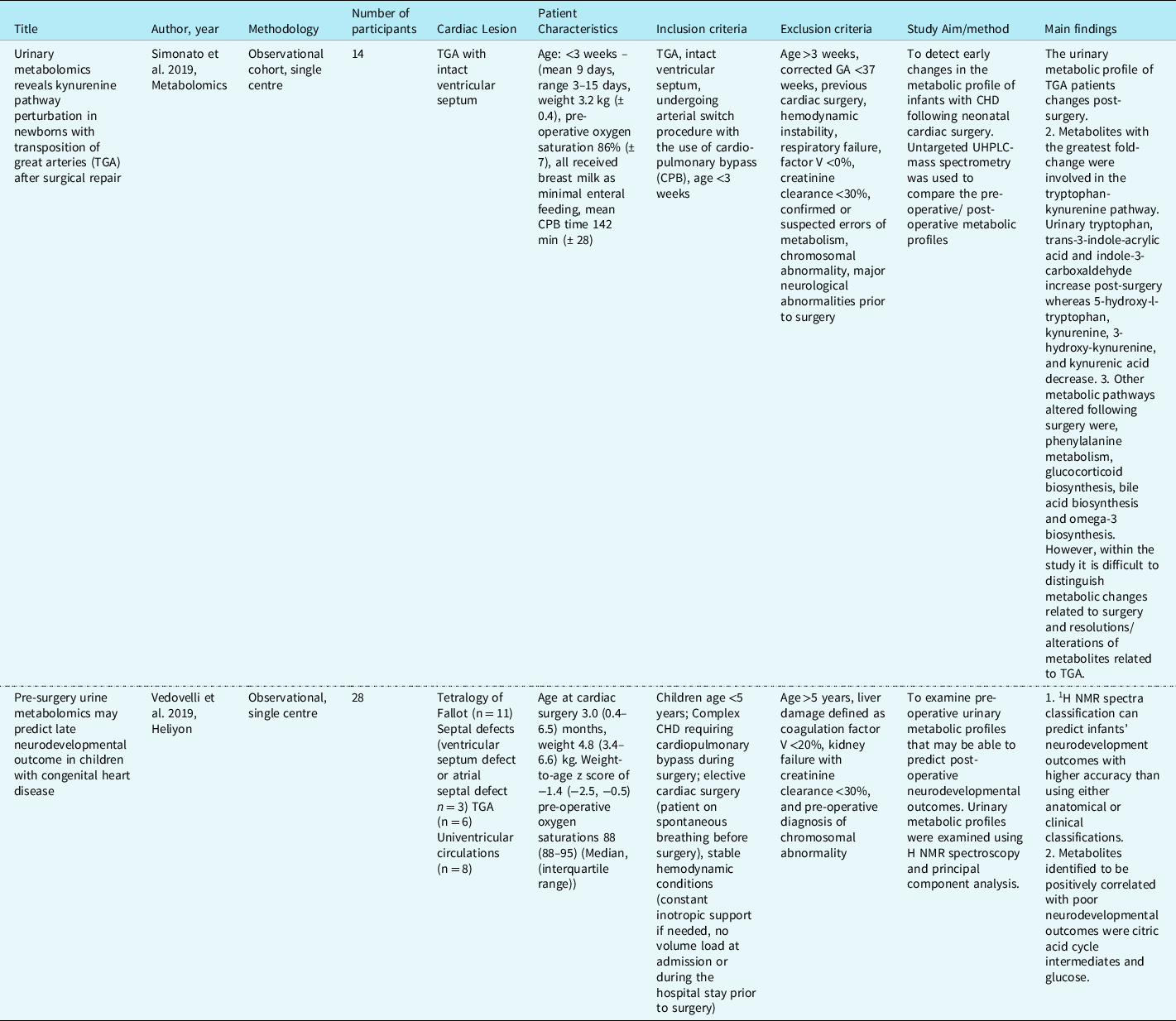
Content analysis: conceptual framework and overarching themes
Content analysis identified two overarching themes relating to metabolic variation predictive of neurodevelopmental abnormalities associated with anaerobic metabolism and metabolic signature associated with the impact on gut microbiota, inflammation, energy, and lipid digestion (Table 2).
Table 2. Metabolites associated with the two overarching themes. Reference Simonato, Fochi and Vedovelli27,Reference Vedovelli, Cogo and Cainelli28

Category 1: Metabolic variation
Both studies characterised cross-sectional patterns of urinary metabolites in infants with CHD. Vedovelli et al. describe changes in metabolites before and after surgery, and a shift to anaerobic metabolism with dysregulated ketone body production was more likely to occur in infants with neurodevelopmental impairment. Reference Vedovelli, Cogo and Cainelli28 The metabolite clusters indicating a shift to anaerobic metabolism were not associated with age, cardiac lesion, or presence or absence of cyanotic heart disease, suggesting that other undescribed factors may be responsible for the observed alteration of the urinary metabolome. Simonato et al. described shifts in metabolites associated with energy production (glycolysis), protein synthesis (phenylalanine and tryptophan (kynurenine) pathways), and fat metabolism following surgery involving cardiopulmonary bypass. Reference Simonato, Fochi and Vedovelli27
Category 2: Metabolic signatures
Both studies characterised cross-sectional patterns of urinary metabolites in infants with CHD. Vedovelli et al.28 described decreases in metabolites post-cardiac surgery. They identified metabolic variation associated with neurodevelopmental abnormalities post-surgery. Such predictive markers included metabolites related to the energy generation such as succinate, lactate, glucose, and sucrose. In infants following surgery using cardiopulmonary bypass, Simonato et al.27 described alterations in the excretion of tryptophan, kynurenine, quinolinic acid, and metabolites related to fatty acid oxidation, inflammation, and oxidative stress.
Discussion
This scoping review has outlined the current understanding of metabolic signatures and their maturation associated with CHD. Due to the paucity of data, it was not possible to draw any conclusions about the relationship between urinary metabolites and growth. Indeed, only two studies were identified describing urinary metabolites in 42 infants with CHD and were included in this scoping review. The findings of these studies characterised changes in the urinary metabolome of infants with CHD before Reference Vedovelli, Cogo and Cainelli28 and following cardiac surgery. Reference Simonato, Fochi and Vedovelli27 Prior to surgery, urinary metabolic profiles were predictive of alterations in energy metabolism (TCA cycle), and the accumulation of intermediaries such as succinate, lactate, and glucose suggested a switch towards anaerobic metabolism. These changes were not associated with age or the presence or absence of cyanosis but were associated with poorer neurodevelopmental outcomes. This suggests that other undescribed factors may influence the observed changes in the urinary metabolome. Reference Vedovelli, Cogo and Cainelli28 Following cardiac surgery, there were marked alterations in the kynurenine pathway of peripheral tryptophan metabolism. This pathway plays a crucial role in the immune response to inflammation, and can either be neuroprotective, neurotoxic or neuro- immunomodulatory, and can also impact on the serotonin pathway with implications for behaviour. Reference Vécsei, Szalárdy, Fülöp and Toldi35 The association of the observed changes in urinary metabolic profiles with neurodevelopmental delay is unclear. Neurodevelopmental abnormalities are common in children born with transposition of the great arteries and other cyanotic lesions, and their aetiology is multifactorial. Reference Miller, Zak and Shrader36 Factors thought to be involved include ischaemia reperfusion injury, Reference Laffey, Boylan and Cheng37 intrauterine cerebral oxygen delivery, or a lack of ketone bodies for the developing brain. Reference Vedovelli, Cogo and Cainelli28
Following surgery involving cardiopulmonary bypass, Simonato et al. identified discriminant metabolites spanning five different pathways including nicotinamide metabolism (including tryptophan, kynurenine) and glycolysis. Reference Simonato, Fochi and Vedovelli27 Correira et al. Reference Correia, Wooi Ng and Wijeyesekera38 analysed serum metabolites during and after cardiac surgery and found that ketone bodies appeared to be associated with improved survival, and observed an association between eight other metabolites (intermediaries of the TCA cycle, muscle metabolism (creatine, creatinine) and fatty acid and ketone body metabolites (acetone, acetoacetate, 3-hydroxybutyrate), and surgical and disease severity. These metabolic derangements may also be affected by other factors such as iron deficiency anaemia, which is common in infants and more so in infants with cyanotic CHD, who are at risk of secondary erythrocytosis. Reference Itiola, Animasahun and Njokanma39 Iron deficiency is known to impact on the TCA cycle as it includes two enzymes that contain Fe-S clusters, aconitase, and succinate dehydrogenase. Reference Mayneris-Perxachs, Amaral and Lubach40 In addition, iron deficiency leads to impaired ketogenosis, decreased citrate synthase and succinate dehydrogenase activity, and thus reduced availability of free fatty acids. Given the important role of free fatty acids and ketone bodies for CNS metabolism, this may have a direct effect on brain development and function. Reference Arisaka, Ichikawa, Imataka, Koyama and Sairenchi41
Importantly neither of the studies included in this scoping review described the impact of these metabolic changes on growth. There are therefore significant gaps in knowledge especially relating to the relationship between an altered urinary metabolome and metabolic maturation, overall and organ-specific growth and neurodevelopmental outcomes, longitudinal changes in the urinary metabolome and energy, protein, and fat metabolism, and metabolic maturation and associations with weight and length gain in infants with complex disease such as CHD compared to a control group of healthy infants.
Evidence that metabolic developmental delay may affect organ growth and maturation is derived from studies of energy expenditure and body composition abnormalities in infants with CHD. Reference Irving, Ravishankar, Miller, Chittams, Stallings and Medoff-Cooper42 It has been shown that there is no difference in resting or total energy expenditure in infants with CHD who had surgery within the first 30 days of life at 3 months and 12 months of age, compared to healthy controls. Reference Trabulsi, Irving and Papas43,Reference Irving, Medoff-Cooper and Stouffer44 However, infants with lower total percentage body fat were more likely to experience growth failure. Reference Irving, Ravishankar, Miller, Chittams, Stallings and Medoff-Cooper42,Reference Trabulsi, Irving and Papas43 Infants with CHD have been shown to have a diminished capacity to metabolise free fatty acids, with higher serum levels of lactate and alanine, Reference Lundell, Sabel and Eriksson45 reduced production of ketone bodies, Reference Amark, Ekroth, Nilsson, Sunnegårdh and Söderberg46 and abnormal fatty acid oxidation and lipid metabolism. These abnormalities may be central to growth retardation; Reference Lundell, Sabel and Eriksson45 however, further work will be required to explore the relationship of these metabolites and growth.
Limitations
This is a scoping review designed to present the current range of evidence specific to urinary metabolites in infants with CHD. It highlights the paucity of longitudinal data describing metabolic maturation. Given this, it was not possible to synthesise results or reliably identify the metabolites associated with growth failure commonly seen amongst infants with CHD despite adequate nutrition support.
Future research priorities
There are considerable gaps in our knowledge relating to how metabolic maturation occurs in infants with CHD, specifically, which factors are associated with metabolic instability and developmental delay. Phenotypes relative to metabolic maturation could be used to inform the development of longitudinal normal phenome-for-age z scores of metabolites of interest to identify metabolic aberrance in infants with CHD with heterogenous disease
Whether future studies targeting metabolites associated with metabolic instability will improve nutrition and growth outcomes in infants with CHD is unknown. Future research is required to describe and define the normal range for urinary metabolites in healthy infants and those with complex disease and of different gestational age to allow the development of phenome age z score charts for metabolites of interest. This might allow the design and testing of age and disease-specific nutritional interventions. In addition, these data may guide refinement of nutrient-energy dense feeds or supplements required for infants with alterations in metabolic maturation to support ideal growth during the first 6 months of life. Reference Mayneris-Perxachs and Swann17,Reference Giallourou, Fardus-Reid and Panic18,Reference Rosa, Mercer and Lin47
Developing a better understanding of this relationship Reference Mayneris-Perxachs and Swann17,Reference Giallourou, Fardus-Reid and Panic18 will help refine our understanding of phenotypic and metabolic responses in infants with CHD and define age-related reference ranges for specific metabolites particularly with regards to developmental delay in the first 6 months of life in order to improve growth outcomes. Reference Giallourou, Fardus-Reid and Panic18–Reference Bourdon, Lelijveld and Thompson21
Conclusion
The results of this scoping suggest that there are considerable gaps in our knowledge relating to metabolic maturation of infants with CHD. Some infants with CHD appear to have aberrant metabolic signatures with an accumulation of TCA intermediaries, with a subsequent downstream effect on lower amounts of ketone bodies available for the developing brain and growth.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951122003262
Acknowledgements
British Dietetic Association General Education Trust Fund for supporting this research and the University of Southampton Library specialists in helping to define the search strategy.
Financial support
This work has received funding from the British Dietetic Association General Education Trust Fund (Ref: 21/05). AY and MJJ are supported by the National Institute for Health Research Southampton Biomedical Research Centre.
Conflicts of interest
None.
Contributors’ statement
Authors made the following contribution to the manuscript: LVM, CW, JA, AY, RMB, JS, MJJ, and JVP formulated the original idea, LVM, CW, JA, and SP completed the database search, data extraction, and analysis, LVM and SP drafted the manuscript, CW, JA, AY, RMB, JS, MJJ, and JVP reviewed and revised the manuscript for important intellectual content, and all authors provided final approval of the version to be submitted.
Appendix
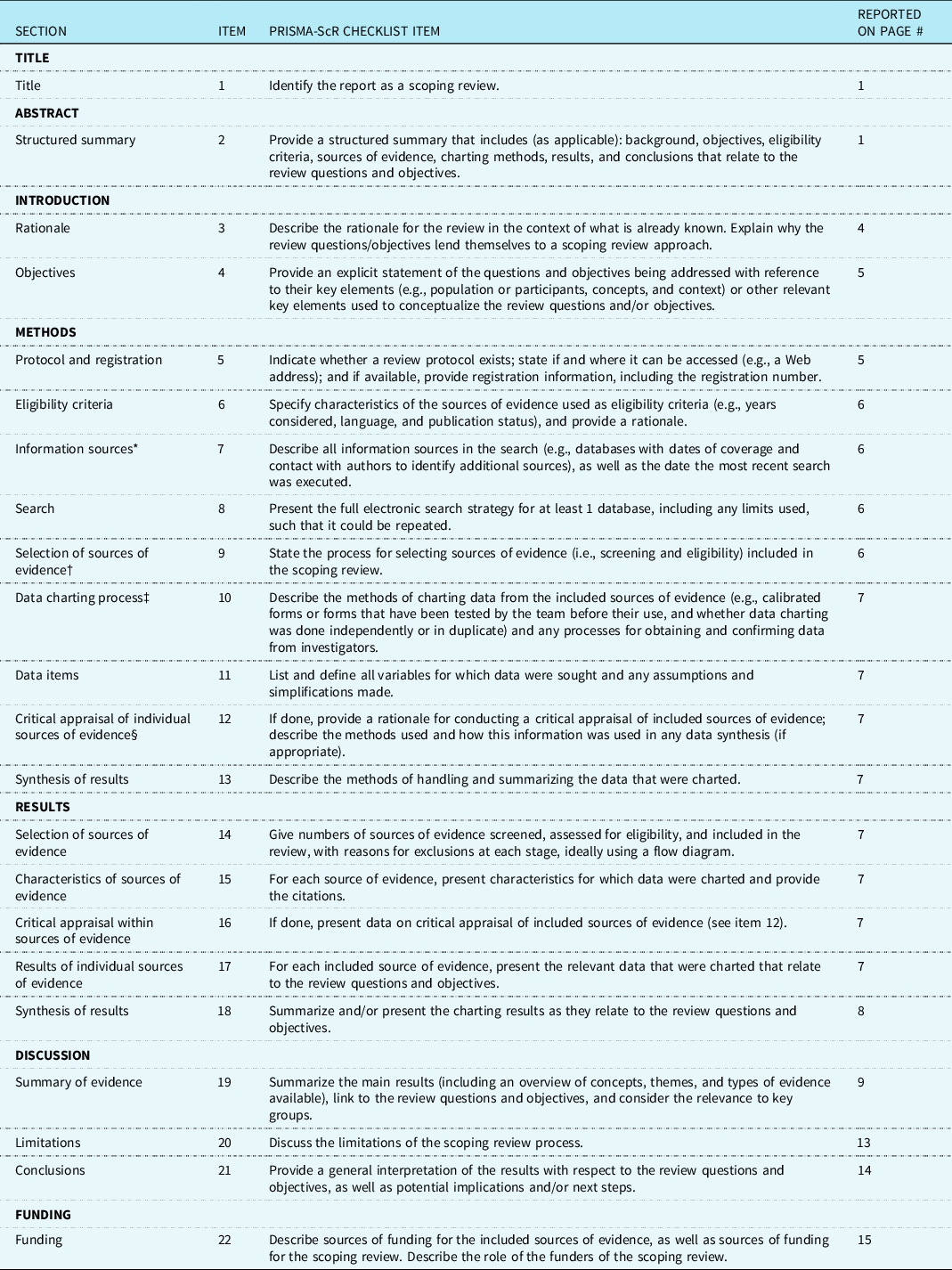
Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist
JBI = Joanna Briggs Institute; PRISMA-ScR = Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews.







