Catheter-related loss of the arterial pulse is one of the most common complications in paediatric cardiac catheterisation. Its frequency in neonates has been reported to be between 19 and 62%. Reference Brotschi, Hug, Kretschmar, Rizzi and Albisetti1–Reference Varan, Yakut, Erdoğan, Özkan and Tokel3 Previous studies have reported variables including younger age, lower weight, prolonged vessel access and procedure times, increased number of attempts, large sheath size, sheath exchange, interventional procedure, access technique, operator experience, activated clotting time <250 seconds, and the diameter of the femoral artery <3 mm for arterial occlusion as risk factors. Reference Brotschi, Hug, Kretschmar, Rizzi and Albisetti1,Reference Song, Choi and Lee4–Reference Bansal, Misra, Forbes and Kobayashi9
Femoral arterial access is challenging, and even in experienced hands, it can be a lengthy procedure in neonates and low-weight infants. Reference Polat2–Reference Song, Choi and Lee4,Reference Lamperti, Biasucci and Disma10,Reference Siddik-Sayyid, Aouad and Ibrahim11 Previous studies have shown that ultrasound-guided vascular access increases the success rate and first attempt success rate and decreases failure rate, number of attempts, vascular access time, and vascular arterial complications. Reference Boran, Urfalıoglu and Arslan5,Reference Alexander, Yohannan and Abutineh7,Reference Lamperti, Biasucci and Disma10–Reference Bruzoni, Slater, Wall, St Peter and Dutta12 Although the evidence level of ultrasound-guided vascular access was classified as 1B for vessel cannulation in adults, no level of evidence has been reported for femoral arterial access in children. Reference Lamperti, Biasucci and Disma10
There are limited data on acute loss of the arterial pulse and permanent femoral arterial occlusion in neonates with CHD. We focused on neonates with CHD who underwent ultrasound-guided femoral arterial access during cardiac catheterisation. We investigated the frequency and risk factors of acute loss of the arterial pulse and permanent femoral arterial occlusion in neonates with CHD placed a 4F sheath.
Methods
We enrolled 323 neonates (≤ 28 days) with CHD who underwent ultrasound-guided femoral arterial access in the catheterisation laboratory at our institution between August, 2017 and May, 2021. Data on ultrasound-guided femoral arterial access and principal variables related the clinical and demographic characteristics of the patients were entered instantaneously by a paediatric cardiologist performing the procedure in our pre-formatted SPSS database. Catheterisation-related adverse events determined during the first 12 weeks of follow-up were enrolled in the same SPSS database at the time of identification. We divided the patients into groups depending on the presence or absence of either acute loss of the arterial pulse or permanent femoral arterial occlusion. The primary aim of the study was to research the frequency and risk factors of acute loss of the arterial pulse and permanent femoral arterial occlusion in neonates with CHD placed a 4F sheath. The secondary aim of the study was to present our single-centre and single-operator experience with arterial access data in neonates with CHD who underwent ultrasound-guided femoral arterial access.
Access technique for femoral artery
We used an echocardiography machine-S6 with 12 L-RS linear vascular probe (General Electric Vivid S6, Oslo, Norway), a 21Gx7 cm beveled ultra sharp needle (Argon Medical Devices, Texas, United States of America) and a 0.018" × 45 cm nitinol guidewire with radiopaque tip of Micro-Stick Introducer Set (Medical Components Inc., Philadelphia, United States of America). Lidocaine (1%) was administered subcutaneously for local anaesthesia. Femoral arterial access was performed on the long axis under general anaesthesia as previously described by Alten et al. Reference Alten, Borasino, Gurley, Law, Toms and Dabal6 We used the same technique and equipments for all attempts during the study period. An experienced paediatric cardiologist (M.G) performed all ultrasound-guided femoral arterial accesses. The operator performed a total of 1460 femoral vascular access during the study. We inserted a 4F sheath (7 cm) (Cordis Corporation, Florida, United States of America). Approximately 5 cm of the 7 cm sheath was placed in patients less than 2.5 kg and full length in the patients more than 2.5 kg.
Diagnosis of loss of the femoral arterial pulse
Haemostasis was achieved through gentle manual compression. The diagnosis of acute loss of the arterial was made based on the evaluation of the cannulated leg for arterial pulse, pallor, and coldness by two paediatric cardiologists at the first hour after bleeding control. 130 (40.2%) of the 323 patients were diagnosed with acute loss of the arterial pulse according to clinical and examination findings and consensus of two paediatric cardiologists. Unfractionated heparin was administered as a loading dose and continued infusion of a maintenance dose in the patients with acute loss of the arterial pulse. We investigated with ultrasound whether pulsatile pulse returned in the patients with acute loss of the arterial pulse whose pulse cannot be obtained at the end of the first, second, and third days after the procedure. We planned the treatment and follow-up of the patients according to the ultrasound findings of the cannulated femoral artery. 19 (5.9 %) of the 323 patients were diagnosed with permanent femoral arterial occlusion according to the ultrasound findings at the end of the 12-week dual treatment.
Protocol of anticoagulant and antiaggregant
After the sheath was placed, we administered intravenous unfractionated heparin 100 U/kg to patients whose < INR 2, and an additional dose of 50 U/kg was administered to patients whose cannulation time was longer than 1 h, as described in previous studies. Reference Alexander, Yohannan and Abutineh7,Reference Tokel, Gümüş, Ayabakan, Varan and Erdoğan13,Reference Knirsch, Kellenberger, Dittrich, Ewert, Lewin and Motz14 Catheters and sheaths were flushed with heparinised saline before use. When acute loss of the arterial pulse was considered in the cannulated leg, unfractionated heparin was administered at a loading dose of 75 U/kg and continued infusion of a maintenance dose of 25 U/kg/hour. Pulses were examined every hour during heparin infusion. The activated partial thromboplastin time value was checked every 6 h to maintain its value between 60 and 80 seconds. In the absence of a normal flow pattern and profile, we began treatment consisting of salicylic acid once at 3–5 mg/kg/day PO and enoxaparin twice at 2–3 mg/kg/day subcutaneously between 44 and 48 h. In the follow-up, antifactor Xa level was checked every 1–2 weeks to maintain its value between 0.5and 1 U/ml. Intravenous thrombolytic treatment was planned in the presence of ischaemia finding in the cannulated leg and persistent thrombus on ultrasonography despite effective anticoagulant therapy.
Definitions
“An attempt” was defined as inserting a needle into the skin and its withdrawal. Repetitive advancement of the needle towards the common femoral artery in the same punctured skin point was not considered as several attempts. “Arterial access time” was defined as the time spent between needle penetration of the skin and placement of the guidewire into the common femoral artery. “Inadvertent puncture” was defined as placement of guidewire in the femoral vein. “Success” was defined as the placement of the sheath into the common femoral artery. The “duration of cannulation” was defined as the time from arterial access to sheath removal. “Acute loss of the arterial pulse” was defined as absent or weak pedal pulses, pallor, and coldness in the cannulated leg in the first hour after bleeding control. “Permanent femoral arterial occlusion” was defined as complete femoral arterial occlusion and collateral circulation development determined by ultrasound despite dual anticoagulant treatment at the end of the 12-week dual treatment.
Statistical analysis
We performed statistical analysis between patients with and without acute loss of the arterial pulse and between patients with and without permanent femoral arterial occlusion. Continuous variables were not normally distributed; therefore, the Mann-Whitney U-test was used to compare variables between independent groups. Chi-square and Fisher’s exact-tests were used to compare dichotomous variables. Multi-variate analysis was performed using logistic regression analysis to determine the independent risk factors of acute loss of the arterial pulse and permanent femoral arterial occlusion. All variables with a p-value < 0.20 in univariate analysis were included in the multi-variate analysis. Hosmer–Lemeshow goodness-of-fit statistics was used to assess model fit. Significant significance was set at a p value < 0.05. Continuous variables were expressed as median (interquartile range [IQR]:25th–75th) and categorical variables were expressed as numbers (percentages). Data input and statistical analyses were performed using IBM SPSS statistical software (version 25.0; IBM Corp. 25.0, Armonk, NY, United States of America).
Results
We achieved successful ultrasound-guided femoral arterial access in 323(98.8%) of 327 neonates who decided to undergo paediatric cardiac catheterisation during the study period. Femoral arterial access could not be obtained in four patients; there were patients with coarctation of the aorta and multiple organ failure and one patient had aortic arch hypoplasia and hip joint deformity. Coarctation angioplasty was performed in two patients in whom we accessed the carotid artery, and two other patients underwent coarctation surgery after CT.
In our cohort, 169 (52.3%) of 323 patients had ductus-dependent circulation. Of 169 patients, 92 were diagnosed with ductus-dependent systemic circulation and 77 patients with ductus-dependent pulmonary circulation. The diagnosis of 92 patients with ductus-dependent systemic circulation were as follows; 29 patients were diagnosed with hypoplastic left heart syndrome, 14 patients with aortic interruption, 12 patients with aortic coarctation, 5 patients with valvular aortic stenosis, and the remaining 32 patients with complex heart disease accompanied by severe arcus hypoplasia. The diagnoses of 77 patients with ductus-dependent pulmonary circulation were as follows; 22 patients were diagnosed with pulmonary atresia with ventricular septal defect, 18 with tetralogy of Fallot, six patients with double outlet right ventricle with severe pulmonary stenosis, one patient with Ebstein’s anomaly, and the remaining 30 patients with complex cyanotic heart with single ventricle physiology. Successful balloon angioplasty was performed in 28 (41.8%) patients with multiple organ failure and discrete coarctation out of 67 patients with aortic coarctation.
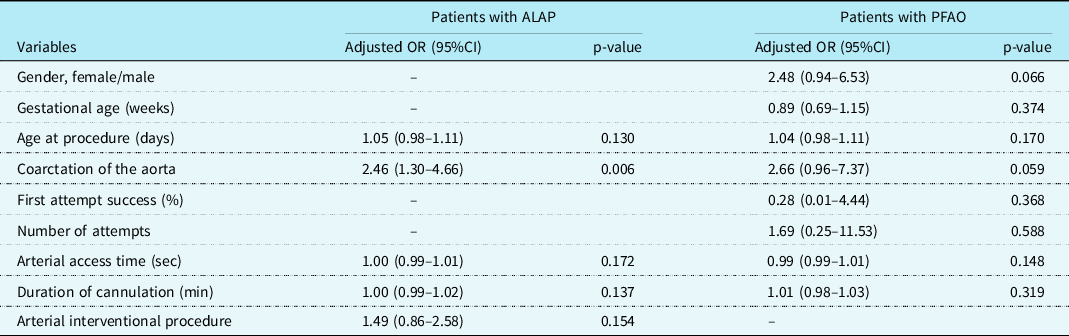
First attempt success was achieved in 285 (88.2%) of the 323 patients. A total of 375 attempts were performed in 323 patients and 13 (4%) patients required ≥3 attempts. The right femoral arterial access was obtained in 192 (59.4%) patients and the left femoral arterial access was achieved in 131(41.6%) patients. The left femoral artery was preferred because right femoral artery spasm developed in 21 of the 131 patients. We determined acute loss of the arterial pulse in 130 (40.2%) of 323 patients at the first hour after bleeding control. Results of Doppler ultrasound and follow-up of patients with loss of femoral artery pulse were shown in detail in figure 1. The characteristics and procedural variables of patients with acute loss of the arterial pulse and results of univariate analysis were summarised in Table 1. As seen in Table 2, logistic regression analysis identified coarctation of the aorta (odds ratio: 2.46; 95% confidence interval: 1.30–4.66; p = 0.006) as independent risk factor for acute loss of the arterial pulse.
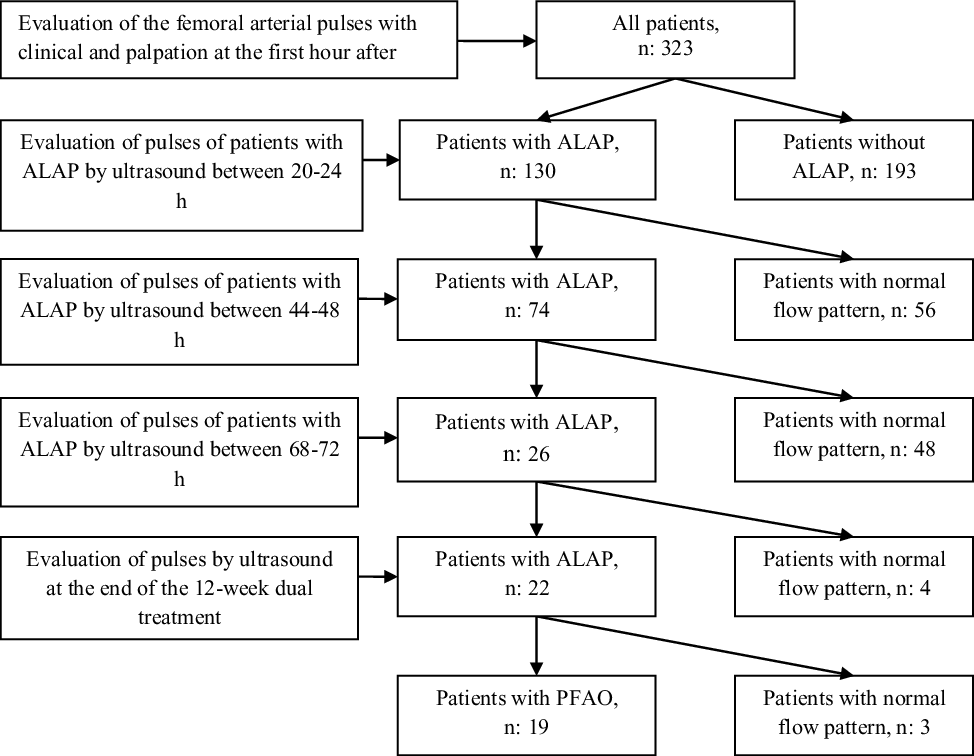
Figure 1. Flow algorithm of the patients with loss of the femoral arterial pulse.
Table 1. Characteristics of patients and procedural variables related to ultrasound-guided femoral arterial access in patients with ALAP.

Abbreviations: ALAP: acute loss of arterial pulse.
Table: 2. Multi-variate analysis of predictors of ALAP and PFAO.
Hosmer and Lemeshow-test’s p values (p: 0.54 for ALAP and p: 0.53 for PFAO, respectively).
Abbreviations: ALAP: acute loss of arterial pulse, PFAO: permanent femoral arterial occlusion.
We performed ultrasound Doppler imaging in 130 patients with acute loss of the arterial pulse who received heparin infusion between 20 and 24 h. There was a normal flow pattern of the common femoral artery in 56 of the 130 patients. We did not demonstrate a normal flow pattern of the common femoral artery in the remaining 74 patients and detected arterial thrombosis in the common femoral artery in 4 of the 74 patients.
The 74 patients with acute loss of the arterial pulse who received heparin infusion were re-evaluated with ultrasound Doppler imaging between 44 and 48 h. There was a normal flow pattern of the common femoral artery in 48 of the 74 patients and we found that thrombi of four patients were revolved. Severe spasm persisted in the remaining 26 patients. Dual treatment consisted of oral salicylic acid and subcutaneous enoxaparin was begun in the 26 patients.
In ultrasound imaging on the third day, normal flow pattern of the common femoral artery was identified in four of the 26 patients with acute loss of the arterial pulse, and dual treatment was continued in the remaining 22 patients. We detected collateral circulation in three patients on the third day. Although arterial thrombosis was not observed in the remaining 22 patients, we continued dual treatment for 12-week dual treatment because arterial occlusion could develop due to the slowing of distal flow. We did not have enough indications for the initiation of fibrinolytic treatment such as ischaemia findings and persistent thrombus in these patients. We detected permanent femoral arterial occlusion and well-developed arterial collateral circulation using ultrasound imaging in the 19 (5.9%) patients, with the exception of three patients at the end of the 12-week dual treatment.
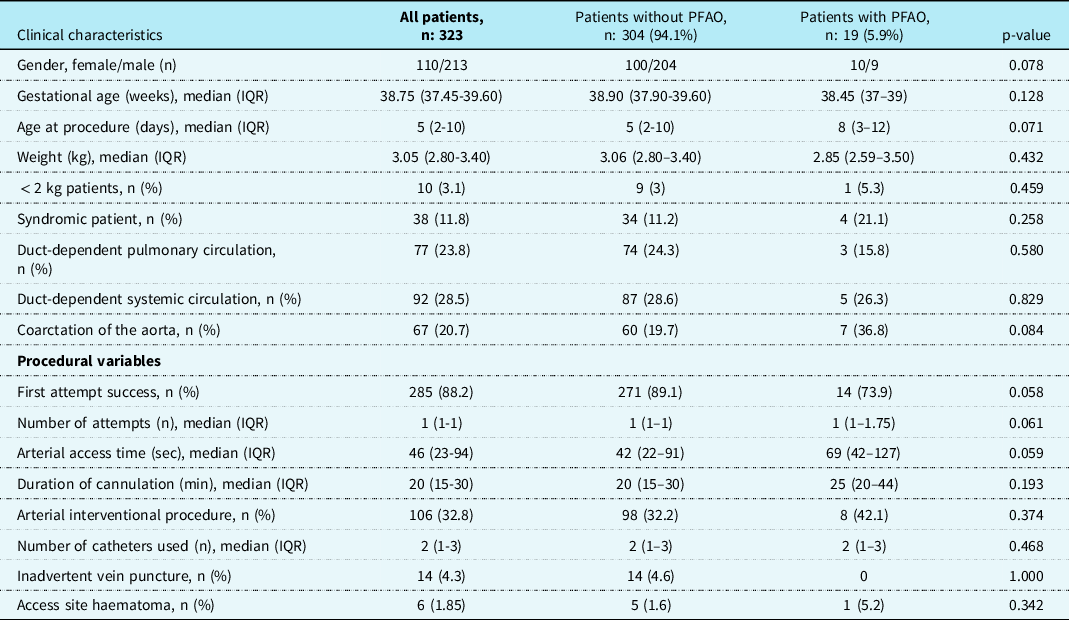
The characteristics and procedural variables of patients with and those without permanent femoral arterial occlusion and results of univariate analysis were summarised in Table 3. As shown in Table 2, logistic regression analysis did not show an independent risk factor for permanent femoral arterial occlusion.
Table 3. Characteristics of patients and procedural variables related to ultrasound-guided femoral arterial access in patients with PFAO.
Abbreviations: PFAO: permanent femoral arterial occlusion.
Haematoma occurred in 6 (1.85%) patients at the access site. Pseudoaneurysm occurred in 2 (0.6%) patients and was surgically repaired. 25(7.7%) patients required transfusion. We did not encounter any case of retroperitoneal haematoma, visceral organ injury, arteriovenous fistula, thromboembolism in the cannulated leg, or procedure-related death.
Discussion
This study presents the largest patient series of ultrasound-guided femoral arterial access in neonates with CHD who underwent paediatric cardiac catheterisation. We identified 130 (40.2%) patients with acute loss of the arterial pulse at the first hour after bleeding control and 19 (5.9%) patients with permanent femoral arterial occlusion at the end of the 12-week dual treatment. We obtained 98.8% successful femoral arterial access, the first attempt success rate of 88.2%, and median arterial access time of 46 sec (IQR: 23–94). As detailed in Table 2, logistic regression analysis identified coarctation of the aorta as an independent risk factor for acute loss of the arterial pulse, but did not identify any independent risk factors for permanent femoral arterial occlusion.
The frequency of acute loss of the arterial pulse can vary according to the time from sheath removal to the evaluation of the pulse. Alexander et al. detected acute loss of the arterial pulse in 33 (6.8%) patients before leaving the catheterisation laboratory, but 30% of these patients had palpable pulse with palpation and ultrasound imaging after an hour, and anticoagulant treatment was commenced according to findings at the first hour. Reference Alexander, Yohannan and Abutineh7 We began anticoagulant treatment for acute loss of the arterial pulse in our patients at the first hour after bleeding control, as in the previous studies. Reference Alexander, Yohannan and Abutineh7,Reference Bansal, Misra, Forbes and Kobayashi9 A loading dose of unfractionated heparin 75 U/kg was administered to patients with acute loss of the arterial pulse. The maintenance dose was initiated at 25 U/kg/h, as in previous studies. Reference Varan, Yakut, Erdoğan, Özkan and Tokel3,Reference Albisetti, Rizzi, Bonduel, Reve-Vilk and Goldenberg8,Reference Tokel, Gümüş, Ayabakan, Varan and Erdoğan13,Reference Bulbul, Galal and Mahmoud15 Collateral circulation can develop in early stages due to increased angiogenesis in neonates. In fact, we detected collateral circulation in three patients on the third day. A study reported that 12.3% of patients had no palpable pulse on discharge despite sufficient time for streptokinase and tissue plasminogen activator treatment. Reference Varan, Yakut, Erdoğan, Özkan and Tokel3 In our cohort, we detected permanent femoral arterial occlusion and well-developed collateral circulation using ultrasound imaging in 19 (5.9%) patients at the end of the 12-week dual treatment. Although our first attempt success rate was quite high, we found a higher (40.2%) acute pulse loss when compared with previous studies. Reference Alexander, Yohannan and Abutineh7,Reference Bansal, Misra, Forbes and Kobayashi9,Reference Knirsch, Kellenberger, Dittrich, Ewert, Lewin and Motz14 This may be due to the low body weight of the cohort.
Femoral arterial diameter in neonates was measured as 2.64 ± 0.85 (1.2–4.5) mm in long axis. Reference Boran, Urfalıoglu and Arslan5 Among patients with arterial diameters of < 3 mm, the frequency of acute loss of the arterial pulse was 25% compared to 1.8% in those with diameters of > 3 mm. Reference Alexander, Yohannan and Abutineh7 Due to the nature of newborn patients, they have risk factors including small age, low body weight, and <3 mm femoral arterial diameter, which are the most important risk factor for femoral artery occlusion. Reference Alexander, Yohannan and Abutineh7,Reference Tokel, Gümüş, Ayabakan, Varan and Erdoğan13,Reference Kulkarni and Naidu16 If we had included patients older than 1 month of age in our study and made a comparison, small age and low body weight could be significant risk factors for acute loss of the arterial pulse and permanent femoral arterial occlusion. Therefore, we found that body weight, age, and gestational age were similar between groups and did not identify them as risk factors for acute loss of the arterial pulse and permanent femoral arterial occlusion.
Several studies have identified the use of a larger sheath as an independent risk factor for arterial occlusion. Reference Knirsch, Kellenberger, Dittrich, Ewert, Lewin and Motz14,Reference Bulbul, Galal and Mahmoud15,Reference Kamyszek, Leraas and Nag17 The incidence of arterial thrombosis in patients that weigh < 5 kg was 5.2% in patients with a 4F sheath and 12.9% in patients with a 5F sheath. Reference Saxena, Gupta, Kumar, Kothari and Wasir18 Because catheter and sheath systems with a lower profile than 4F were unavailable in our country, we placed a 4F sheath in the patients during the study and did not perform a comparison about the sheath size.
Interventional procedures were performed in 32.8% of our patients. In the univariate analysis, interventional procedure was statistically significant in patients with acute loss of the arterial pulse than in those without acute loss of the arterial pulse, but there was no difference between patients with and those without permanent femoral arterial occlusion. In the multi-variate analysis, interventional procedures were not found to be independent risk factors in patients with acute loss of the arterial pulse and permanent femoral arterial occlusion and this result was similar to the findings of previous studies. Reference Alexander, Yohannan and Abutineh7,Reference Glatz, Shah and McCarthy19 We primarily preferred non-invasive diagnostic tools such as echocardiography and cardiac computerised tomography for cardiac diagnosis. Non-invasive diagnostic methods and the use of 3.3 Fr sheaths in diagnostic catheterisation procedures may reduce the incidence of femoral arterial occlusion.
Several studies have reported that prolonged cannulation time increases arterial occlusion and access site complications. Reference Tokel, Gümüş, Ayabakan, Varan and Erdoğan13,Reference Kamyszek, Leraas and Nag17,Reference Saxena, Gupta, Kumar, Kothari and Wasir18 In our cohort, the cannulation time was significantly shorter than in previous studies. Reference Alexander, Yohannan and Abutineh7,Reference Tokel, Gümüş, Ayabakan, Varan and Erdoğan13,Reference Bulbul, Galal and Mahmoud15 The cannulation time was statistically longer in patients with acute loss of the arterial pulse than in those without acute loss of the arterial pulse. No significant difference was found between patients with and those without permanent femoral arterial occlusion. Although the cannulation time was found to be significantly longer in patients with acute loss of the arterial pulse in the univariate analysis, we did not identify cannulation time as a risk factor for patients with acute loss of the arterial pulse and permanent femoral arterial occlusion in the multi-variate analysis.
Although the number of attempts, posterior wall puncture and inadvertent vessel access are reduced in-plan technique compared out-plane tecnique, Reference Song, Choi and Lee4–Reference Alten, Borasino, Gurley, Law, Toms and Dabal6 there is no significant difference in overall success rate between the two techniques. Reference Song, Choi and Lee4,Reference Boran, Urfalıoglu and Arslan5 In-plane technique, we can detect the relationship between the needle tip and the anterior wall and the advancement of the needle and guidewire in the arterial lumen. The needle and the femoral artery are in the same plan and there is no vein in the image. Reference Alten, Borasino, Gurley, Law, Toms and Dabal6 Posterior wall puncture and inadvertent access are associated with increased access site complications. Therefore, we have routinely used in-plane technique for femoral arterial access in our catheterisation laboratory. Haematoma and arteriovenous fistula are among the complications of inadvertent vascular puncture. In previous studies, inadvertent puncture was reported in 5–7% of patients in the ultrasound group and in 23–31% of patients in landmark group. Reference Alten, Borasino, Gurley, Law, Toms and Dabal6,Reference Bruzoni, Slater, Wall, St Peter and Dutta12 Inadvertent vein puncture occurred in 4.3% of our patients. While haematomas were seen in 6 (1.85%) patient, no arteriovenous fistula was identified in our study. We found that the incidences of haematoma and inadvertent puncture were quite low when compared to a study of neonate with femoral arterial access. Reference Boran, Urfalıoglu and Arslan5,Reference Alten, Borasino, Gurley, Law, Toms and Dabal6,Reference Bruzoni, Slater, Wall, St Peter and Dutta12
The first attempt success in ultrasound-guided femoral arterial access was reported to be between 25 and 53% in neonates. Reference Polat2,Reference Boran, Urfalıoglu and Arslan5 We obtained a high success rate of 98.8% and a very high first attempt success rate of 88.2% compared to previous studies. Reference Polat2,Reference Boran, Urfalıoglu and Arslan5,Reference Alten, Borasino, Gurley, Law, Toms and Dabal6,Reference Siddik-Sayyid, Aouad and Ibrahim11,Reference Law, Borasino, McMahon and Alten20 There was no significant difference in first attempt access between patients with and without acute loss of the arterial pulse and between patients with permanent femoral arterial occlusion and those without permanent femoral arterial occlusion. A high first attempt access results in a shorter arterial access time and a minimum number of attempts. An increased number of attempts causes an increase in the frequency of adverse events. Reference Polat2,Reference Alten, Borasino, Gurley, Law, Toms and Dabal6,Reference Saxena, Gupta, Kumar, Kothari and Wasir18 The median number of attempts in all our patients was 1 (IQR: 1–1) and was satisfactory compared to the findings of previous studies. Reference Polat2,Reference Boran, Urfalıoglu and Arslan5,Reference Alten, Borasino, Gurley, Law, Toms and Dabal6 There was no difference in the number of attempts between patients with and those without acute loss of the arterial pulse and between patients with and those without permanent femoral arterial occlusion.
In a study where 65 neonates were included in whom only the arterial cannula was placed for the purpose of arterial monitoring and no guidewire or sheath were placed, the mean arterial access time was reported to be 74 ± 45 sec on long axis. Reference Boran, Urfalıoglu and Arslan5 In a study that compared 0.014-inch and 0.019-inch guidewires used during ultrasound-guided femoral arterial access in 124 neonates, arterial access time was reported to be 471 ± 59 sec in the 0.014-inch group and 549 ± 79 sec in the 0.019-inch group. Reference Polat2 In our study, the median access time was significantly longer in patients with acute loss of the arterial pulse than in those without acute loss of the arterial pulse. However, no significant difference was found between patients with and those without permanent femoral arterial occlusion. We obtained a shorter time in terms of arterial access time compared to those of studies that used traditional techniques and ultrasound-guided femoral arterial access. Reference Polat2,Reference Boran, Urfalıoglu and Arslan5,Reference Alexander, Yohannan and Abutineh7,Reference Tokel, Gümüş, Ayabakan, Varan and Erdoğan13,Reference Bulbul, Galal and Mahmoud15,Reference Law, Borasino, McMahon and Alten20 Although a study reported that long access time increases the complications of the access site, Reference Tokel, Gümüş, Ayabakan, Varan and Erdoğan13 our study did not identify arterial access time as a risk factor in patients with acute loss of the arterial pulse and permanent femoral arterial occlusion.
Because CHD with cyanotic and left right shunts had no effect on the clinic status in the neonatal population, we divided cardiac pathologies into groups according to the presence of duct-dependent circulation and coarctation of the aorta. We did not identify a statistical relationship between duct-dependent circulations in patients with acute loss of the arterial pulse and in those with permanent femoral arterial occlusion. In univariate analysis, coarctation of the aorta was statistically significant in the patients with acute loss of the arterial pulse than in those without acute loss of the arterial pulse, but there was no difference between the patients with and those without permanent femoral arterial occlusion. In multi-variate analysis, logistic regression analysis identified coarctation of the aorta as an independent risk factor for acute loss of the arterial pulse, but did not identify any independent risk factors for permanent femoral arterial occlusion. To our knowledge, this is the first study to report coarctation of the aorta as an independent risk factor for acute loss of the arterial pulse in neonates with CHD.
Study limitations
This study has several limitations. First, lack of data regarding pre-access femoral artery measurements and the overlap status of vessels. Second, we did not obtain the frequencies of arterial posterior puncture during arterial access. We used ultrasound-guided femoral arterial access in the long axis approach, which has the lowest posterior wall puncture compared to other techniques. Third, for some reasons, the vessel diameter, spasm severity, acute loss of the arterial pulse, and the presence of thrombosis in our patients were not evaluated using ultrasound before anticoagulant treatment. We evaluated the femoral artery in several patients who underwent physical examination and ultrasound immediately after bleeding control at the beginning of our study. We did not detect a palpable pulse and found severe arterial spasm and monophasic waveform using ultrasound in these patients. After bleeding was controlled, the patients were transferred to the pediatric cardiac intensive care unit. Cannulated femoral arteries were re-evaluated by ultrasound for acute loss of the arterial pulse at the first hour after bleeding control. During ultrasound imaging of the femoral artery, ultrasound imaging caused significant heat loss, resulting in re-bleeding and haematoma formation due to the opening of the dressing in two patients. In addition, we evaluated the patients’ pedal pulses with ultrasound Doppler for the decision of acute loss of the arterial pulse but did not obtain reliable quality flow pattern, flow profile, and flow velocities from pedal pulses measurements. The patients were diagnosed with acute loss of the arterial pulse according to clinical and examination findings and consensus of two pediatric cardiologists. Fourth, our study was a single operator, single-centre study. If it had been a multi-centre study, differences in the level of experience and technique could have affected the results.
Conclusion
Our study shows that acute loss of the arterial pulse and permanent femoral arterial occlusion are high and important problems despite the early initiation of anticoagulant treatment. Coarctation of the aorta was identified as an independent risk factor for acute loss of the arterial pulse. Ultrasound-guided femoral arterial access improves arterial access variables, including overall success rate, first attempt success, number of attempts, time to access time, and inadvertent vein puncture when compared with those of previous studies. Reference Song, Choi and Lee4,Reference Alten, Borasino, Gurley, Law, Toms and Dabal6,Reference Siddik-Sayyid, Aouad and Ibrahim11,Reference Law, Borasino, McMahon and Alten20 Ultrasound-guided femoral arterial access on long axis is safe and feasible in elective and emergency clinical settings. Multi-centre studies with large series of neonates are needed to establish the appropriate time of diagnosis, diagnostic tools, treatment protocols, and follow-up.
Acknowledgements
We would like to thank the cardiac catheterization laboratory technicians for their assistance with arterial access and collection of access variables.
Financial support
This study was supported by Baskent University Research Found.
Conflicts of interest
None
Ethical standards
This study was approved by Baskent University Institutional Review Board (Project no: KA21/238).







