Anomalous left coronary artery from the pulmonary artery is a rare congenital heart malformation, which occurs in about 1 in 300, 000 newborns, accounting for about 0.24%–0.46% of CHD.Reference Yujuan and Peijun1 Anomalous left coronary artery from the pulmonary artery is usually isolated, but a small proportion of anomalous left coronary artery from the pulmonary artery is associated with cardiac macrovascular malformations such as ventricular septal defect, patent ductus arteriosus, tetralogy of Fallot, pulmonary stenosis, and aortic coarctation, which may have important functional significance. For example, combined patent ductus arteriosus or ventricular septal defect both deliver blood with higher oxygen saturation directly to the anomalous left coronary artery from the pulmonary artery, and if the presence of anomalous left coronary artery from the pulmonary artery is not detected before operation, direct ligation of the patent ductus arteriosus or closure of the ventricular septal defect will have fatal consequences. In 1993, Bland, White, and Garland described the clinical manifestations and autopsy results of a 3-month-old child in detail for the first time and made the diagnosis of the disease for the first time, so anomalous left coronary artery from the pulmonary artery is also called Bland-White-Garland syndrome.Reference Kudumula, Mehta and Stumper2 Left ventricular dysfunction, myocardial infarction, and/or severe mitral regurgitation due to coronary “steal” are associated with extremely high mortality in infancy if not treated surgically in time.Reference Haijiang, Jian and Jimei3 About 90% of children die within the first year of life. At present, double coronary system reconstruction is the standard procedure for anomalous left coronary artery from the pulmonary artery, but the surgical methods of double coronary system reconstruction are endless, and the long-term follow-up results are different. Whether to treat mitral regurgitation at the same time is still controversial. There is no standard surgical procedure for anomalous left coronary artery from the pulmonary artery with left intramural coronary artery. The present review specifically addresses these aforementioned concerns.
Pathophysiology and classification
EdwardsReference Edwards4 is credited with being the first to provide a pathophysiological explanation of coronary flow in patients with anomalous left coronary artery from the pulmonary artery. The onset of symptoms and the degree of myocardial ischaemia depend on the rapid degree of closure of the patent ductus arteriosus and the maintenance of a balance of pulmonary hypertension, as well as the establishment of intercoronary collateral vessels, to provide retrograde perfusion from the right coronary artery to the anomalous left coronary artery from the pulmonary artery. During the neonatal period and early infancy, the left coronary artery maintains sufficient perfusion pressure to perfuse the myocardium, since the pulmonary artery pressure has not yet fallen, as has the aorta. As the newborn grows, the decreased pulmonary artery pressure leads to a reduction in the blood supply to the left coronary artery. The tolerance of left ventricular myocardium depends entirely on the abundance of collateral circulation between the coronary arteries. At this time, anomalous left coronary artery from the pulmonary artery can be divided into infantile and adult types according to the degree of collateral coronary steal and improved myocardial perestablishment.Reference Ma, Wang and Hua5
In infantile anomalous left coronary artery from the pulmonary artery, at 8 weeks after birth, due to the decrease in pulmonary artery pressure, there is a lack or only a few collateral branches between the left and right coronary arteries, resulting in significant insufficiency in the coronary artery blood supply area, which is clinically manifested by congestive heart failure and secondary mitral regurgitation. At this time, the clinical manifestations of children are mainly atypical clinical symptoms such as growth retardation, hyperhidrosis, and dyspnoea, while echocardiography shows obvious dilatation of the left heart and poor systolic function of the heart. Such children’s conditions progress rapidly, and about 90% of children die within 1 year of age if not treated in time.Reference Chang-wei, Shou-jun and Hao6 Adult anomalous left coronary artery from the pulmonary artery establishes rich collateral circulation between the left and right coronary arteries within a few weeks after birth. At this time, blood from the right coronary artery enters the left coronary artery through the collateral branches and then returns to the pulmonary artery, which is called steal syndrome.Reference Muzaffar, Ahmad, Gpoal and Wani7 Such patients survive for a long time because of the relatively sufficient blood supply of the left heart, but the blood flow through the left coronary artery is directly distributed to the pulmonary artery due to low pulse pressure and other factors, instead of the myocardial vessels. This long-term left-to-right shunt and steal syndrome will lead to a slow ischaemic change in the blood supply of the left heart. Such chronic myocardial ischaemia can lead to mitral regurgitation, significant heart enlargement and malignant arrhythmia, and eventually lead to the death of patients.Reference Kazmierczak, Ostrowska, Dryzek, Moll and Moll8
In addition to the classification of infantile and adult types, Smith and colleaguesReference Smith, Arnold and Anderson9 proposed a descriptive classification in 1989, describing various patterns of coronary artery origin and their possible surgical implications, which can help in the selection of intraoperative surgical strategies. The specific anomalous left coronary artery from the pulmonary artery classification is shown in Table 1 and Figure 1.

Figure 1. Diagrammatic representation of aortic and pulmonary artery origins of left coronary artery in normal and anomalous conditions. Cephalic views depict aperson in the nonfacing sinus with the right hand always signifying sinus 1 and the left hand always signifying sinus 2.
Table 1. Anatomical classification of anomalous left coronary artery from the pulmonary artery

Rule: Facing the aorta and viewed from the pulmonary valve nonfacing sinus, the pulmonary valve sinus was named the right side (sinus 1) and the left side (sinus 2). Sinus 1 of the aorta is opposite sinus 2 of the pulmonary trunk and vice versa. The two nonfacing sinuses are then located at the farthest ends.
Diagnosis
Anomalous left coronary artery from the pulmonary artery is clinically characterised by hyperhidrosis, dyspnoea, developmental arrest, and atypical angina. Most patients present with moderate to severe congestive heart failure, cardiomegaly on chest X-ray, ischaemic findings on electrocardiogram, and a murmur of mitral regurgitation on auscultation. At present, the most conventional method for the diagnosis of anomalous left coronary artery from the pulmonary artery is transthoracic echocardiography, which can find the obvious enlargement of the heart, with or without severe mitral regurgitation, and can also find the obvious enlargement of the right coronary artery, and the left coronary artery originating from the pulmonary artery or its branches. With the development of echocardiography, experienced doctors can easily detect anomalous left coronary artery from the pulmonary artery. Echocardiography has gradually replaced coronary angiography and CT in the diagnosis of anomalous left coronary artery from the pulmonary artery.Reference Schwartz, Jonas and Colan10
Indications for surgery
The early stage of infantile anomalous left coronary artery from the pulmonary artery is due to ischaemic heart failure, and the mortality rate within one year is as high as 90% in patients without operation. Even though adult anomalous left coronary artery from the pulmonary artery patients have abundant collateral branches, the malignant arrhythmia caused by chronic heart failure and then sudden death occurs in 80%–90% of patients over 35 years old. Therefore, no matter what type of anomalous left coronary artery from the pulmonary artery patients, once the diagnosis is confirmed in theory, even asymptomatic patients, regardless of the collateral circulation between the left and right coronary arteries, should immediately undergo surgical treatment to promote the recovery of left ventricular function.
Surgical strategy and whether to treat mitral regurgitation simultaneously
The purpose of surgical treatment of anomalous left coronary artery from the pulmonary artery is to eliminate the phenomenon of left coronary artery steal, restore the normal blood supply of left coronary artery, avoid further myocardial damage, and promote the recovery of left ventricular function. Therefore, early restoration of dual coronary circulation is the guiding goal of surgery. At present, whether anomalous left coronary artery from the pulmonary artery surgery requires concomitant treatment of mitral regurgitation remains controversial. Some scholarsReference Caspi, Pettitt, Sperrazza, Mulder and Stopa11,Reference Lange, Vogt and Horer12 believe that mitral regurgitation in patients with anomalous left coronary artery from the pulmonary artery is related to ischaemic left ventricular dilatation, annular enlargement, and papillary muscle dysfunction. After restoring dual coronary artery blood supply, left ventricular function is improved and the degree of mitral regurgitation is reduced. There is no evidence that mitral regurgitation is related to early death. In order to shorten the aortic cross-clamp time, simultaneous treatment of mitral valve disease is not recommended regardless of the severity of mitral regurgitation. Related studies have shown that for patients with mitral regurgitation, mitral regurgitation was significantly reduced after repair of the anomalous left coronary artery,Reference Ginde, Earing, Bartz, Cava and Tweddell13 and for patients who underwent mitral valve repair at the same time, mitral regurgitation was also significantly reduced. However, the postoperative follow-up of patients with severe mitral regurgitation indicated that some patients still had mild to moderate mitral regurgitation.Reference Muzaffar, Ahmad, Gpoal and Wani7 For adult anomalous left coronary artery from the pulmonary artery, most scholars believe that secondary mitral regurgitation does not need surgical treatment, because there is no significant improvement in valve regurgitation after early valvuloplasty, and early mild to moderate mitral regurgitation is acceptable. However, for patients with mitral regurgitation and heart failure, mitral valve management is difficult and will increase myocardial ischaemia time. At the same time, they believe that infantile mitral regurgitation does not need surgical treatment even if it is severe.
On the contrary, other scholars believe that mitral valvuloplasty can help reduce early postoperative mitral regurgitation, improve cardiac function, and reduce the reoperation rate due to mitral regurgitation. Therefore, they believe that surgical treatment for mitral regurgitation is advocated.Reference Weixler, Zurakowski and Baird14,Reference Neumann, Sarikouch and Bobylev15 Kudumula et al.Reference Thomas, Chan, Alsoufi, Vinocur and Kochilas16 suggested that only the structural mitral valve should be treated. In their report of 25 patients with anomalous left coronary artery from the pulmonary artery, 19 patients had moderate or severe mitral regurgitation, only four patients with structural mitral valve disease underwent mitral valvuloplasty at the same time, and the median follow-up time was 8 years. There was no late death, and only four patients had moderate mitral regurgitation. Amanda S. Thomas et al.Reference Thomas, Chan, Alsoufi, Vinocur and Kochilas16 found in the long-term follow-up of 228 children with anomalous left coronary artery from the pulmonary artery who underwent surgical correction, among patients with moderate or severe mitral regurgitation, that the risk of death was 28% lower when undergoing mitral valvuloplasty. The heart team of Shanghai Children’s Medical CenterReference Kai, Jinghao and Wei17 summarised and evaluated the experience of surgical treatment of anomalous left coronary artery from the pulmonary artery patients in their institution for 15 years. Among the 126 patients, 72 patients with severe or severe mitral regurgitation underwent simultaneous mitral valvuloplasty. Mitral regurgitation was maintained below moderate in 89.2% of patients after mitral valvuloplasty. The flow velocity of mitral valve after mitral valvuloplasty was not significantly increased compared with that before mitral valvuloplasty, and the risk of reoperation was not increased. The team of Guangdong Provincial People’s HospitalReference Haijiang, Jian and Jimei3 summarised the efficacy of left coronary artery replantation in 52 anomalous left coronary artery from the pulmonary artery patients in their team. Mitral valvuloplasty was performed in 16 patients (30.8%) with moderate-severe or severe mitral regurgitation. The results showed that the degree of postoperative mitral regurgitation was significantly reduced compared with that before operation. Although there was no further improvement during the follow-up period, the effect was good, only one patient underwent mechanical mitral valve replacement 2 years after operation because of aggravating mitral regurgitation.
The authors have retrospectively analysed the clinical data of 89 children with anomalous left coronary artery from the pulmonary artery who underwent surgical treatment in Beijing Children’s Hospital from January 2007 to January 2022 and published the relevant follow-up results.Reference Wang, Ding, Zhang, Zhu, Li and Li18 Seven patients underwent extracorporeal membrane oxygenation support for acute left heart failure after operation, and two patients were discharged after weaning successfully. Eight patients died in the early postoperative period, all of them were infants, of which five patients underwent extracorporeal membrane oxygenation support, two patients died of cerebral haemorrhage, two patients died of multiple organ dysfunction, and four patients died of left heart failure. Three patients died late, three patients were lost to follow-up, and 78 patients (96.3%) completed long-term follow-up. A logistic regression model multivariate analysis showed that postoperative moderate or severe mitral regurgitation (OR 26.948 p = 0.024) and prolonged aortic cross-clamp time (OR 1.038 p = 0.050) were independent risk factors of early mortality. Compared with the non-mitral valvuloplasty group (20/36), the mitral valvuloplasty group (patients with moderate or severe mitral regurgitation who underwent mitral valvuloplasty at the same time) (16/36) had more significant improvement in early postoperative left ventricular ejection fraction(LVEF) [(50.68 ± 13.85)% versus (40.50 ± 13.58)% p = 0.033] and had a lower proportion of moderate or severe mitral regurgitation after operation (2/16 versus 11/20 p = 0.014). Children with anomalous left coronary artery from the pulmonary artery can obtain a good prognosis by reconstructing the blood supply of both coronary arteries. Mitral valvuloplasty is more helpful in improving the prognosis of children with moderate or severe mitral regurgitation and mitral valve structural disease. Reasonable placement of extracorporeal membrane oxygenation can help reduce the mortality of critically ill children after operation, but be alert to complications in the central system. In conclusion, the authors believe that for infants with moderate to severe mitral regurgitation before operation, the restoration of coronary artery anatomical circulation does not rapidly improve the function of the mitral valve and affects the recovery of cardiac function early after operation. Therefore, our experience suggests that no matter whether functional or structural abnormalities, moderate or severe mitral regurgitation should be treated at the same time as anomalous left coronary artery from the pulmonary artery anatomical repair of mitral regurgitation. For patients with significant cardiac function decline, it is necessary to carefully evaluate the cardiac function of the patient by multi-disciplinary team, formulate detailed plans, predict the risk encountered, protect the cardiac function during the operation, strictly control the perfusion time, etc. When postoperative cardiac function is poor, cardiac assist technology such as extracorporeal membrane oxygenation and balloon counterpulsation should be used as early as possible to help patients gradually recover cardiac function.
Surgical methods
Reconstruction of the single coronary system
It mainly refers to ligating the left coronary artery originating from the pulmonary artery, which is suitable for patients with rich collateral circulation and large left-to-right shunt. The procedure reduced coronary steal and improved myocardial perfusion. However, pure right coronary perfusion does not conform to normal physiological circulation, chronic left ventricular ischemia still exists, and single coronary circulation is prone to coronary atherosclerosis, with a sudden death risk of up to 33%. At present, the operation is only suitable for emergency palliative surgery in children with severe symptoms, in order to reduce the time of myocardial ischaemia as much as possible. As shown in Figure 2.
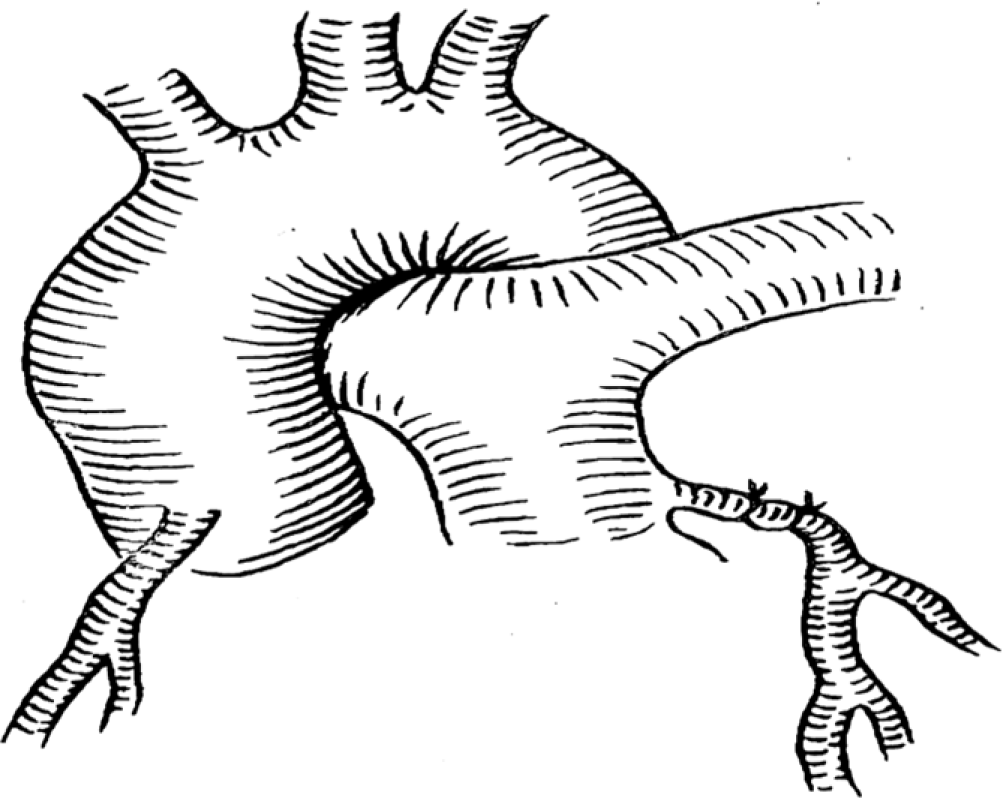
Figure 2. Left coronary artery was ligated.
Reconstruction of dual coronary system
In the Takeuchi procedure, an artificial main pulmonary artery window was constructed, and then a channel was created in the pulmonary artery lumen by using the pulmonary artery wall or autologous pericardium. The aerobic blood flow from the aorta was transferred to the left coronary artery through the channel.Reference Takeuchi, Imamura and Katsumoto19 This procedure is mainly suitable for the anomalous left coronary artery originating from the left lateral or left posterior wall of the pulmonary artery, but there are many long-term complications. As shown in Figure 3.

Figure 3. A tunnel of coronary flow was constructed between the aorta and the ectopic left coronary artery by using a pulmonary artery wall flip sheet. The pulmonary artery was then reconstructed with the autologous pericardium.
Coronary artery bypass grafting, which involves ligation of the left coronary artery and end-to-end anastomosis of the aortic root to the left coronary artery via a dacron vessel, great saphenous vein, left subclavian artery or radial artery. This procedure is mainly suitable for the reconstruction of dual coronary artery system after coronary artery ligation.Reference Naimo, Fricke and D’Udekem20 This is shown in Figure 4.

Figure 4. a: Left subclavian artery was anastomosed with left coronary artery (LCA). b、c、d: After ligation of the LCA, end-to-end anastomosis was performed by connecting the aortic root to the LCA through the great saphenous vein or radial artery.
Left coronary artery reimplantation is considered to be the best surgical method for the treatment of anomalous left coronary artery from the pulmonary artery. Left coronary artery is mainly transplanted to the aortic root, and in the early stage it is mainly suitable for coronary artery originating from the right lateral or posterior wall of the pulmonary artery. This is shown in Figure 5. However, with the progress of coronary artery reimplantation technology, this operation is currently suitable for all patients with anomalous left coronary artery from the pulmonary artery, and more and more modified surgical methods have been adopted, with few long-term complications and good prognosis.Reference Toumpourleka, Belitsis, Alonso, Rubens, Moat and Gatzoulis21 Different surgical methods should be selected according to different anatomical characteristics. For children with short left coronary artery and the opening is close to the aortic trunk, the bay window technique can be used to directly graft the coronary artery to the aortic root. For patients with a long distance from the left coronary artery opening to the aorta, autologous tissue-stitched conduits (pulmonary artery conduit, pericardial conduit and double flap conduit) were used to extend the coronary artery and reduce anastomotic tension.

Figure 5. Classic left coronary artery reimplantation procedure.
Bay window technique
After transection of the main pulmonary artery, the pulmonary artery patch containing the opening of left coronary artery was cut from the main pulmonary artery, and left coronary artery was fully freed. The flap incision was made at the corresponding position of the aortic root, and the coronary artery patch was directly anastomosed with the aortic incision. Attention should be paid to the direction and angle of coronary artery anastomosis to avoid torsion or angulation resulting in postoperative coronary hypoperfusion. As shown in Figure 6.

Figure 6. The wall of the aortic sinus was incised in an “L” shape, and the “bay window” was anastomosed with left coronary artery.
Autologous tissue coronary artery conduit
The pulmonary artery tissue conduit was similar to the bay window technique. First, a large sleeve pulmonary artery patch containing the opening of the left coronary artery was cut from the pulmonary artery trunk, and the upper edge of the patch was turned inward to make edge-to-edge anastomosis to the lower edge to form the left coronary trunk. This is shown in Figure 7. Autologous pericardium tissue can also be used as a conduit to extend the left main coronary artery. The specific operation is to cut off the left coronary artery opening patch, turn over and suture a large piece of autologous pericardium to form a hollow conduit, and extend the left coronary artery opening and connect to the aortic root valve sinus. Double flap conduit was originally conceived for anomalous left coronary artery from the pulmonary artery repair.Reference Cabrera, Chen and Pignatelli22 As shown in Figure 8.

Figure 7. The coronary conduit was extended using the autologous pulmonary artery wall.

Figure 8. Double flap conduit.
Intramural coronary course refers to the course of a segment of the coronary artery within the aortic wall, which is not a rare congenital coronary artery anomaly. It is more common in cases where the coronary artery originates from the abnormal aortic sinus, and it is usually treated by unroofing the intramural segment of the coronary artery.Reference Brothers, Frommelt, Jaquiss, Myerburg, Fraser and Tweddell23 However, anomalous left coronary artery from the pulmonary artery with intramural left coronary artery is a very rare subtype of anomalous left coronary artery from the pulmonary artery, and its surgical management is more challenging, and there is still a lack of standard surgical methods. See Figure 9. Three different surgical methods were used to treat anomalous left coronary artery from the pulmonary artery with intramural left coronary artery in our centre. A total of 10 patients were enrolled, including eight males and two females, with a median age of 7.5 (3–46) months at surgery. The surgical procedures included coronary unroofing in seven cases, coronary unroofing + coronary replantation in two cases, coronary unroofing + left coronary artery ostium closure in one case. A total of seven patients underwent other operations at the same time, including mitral valvuloplasty in five patients. Delayed sternal closure occurred in two cases. There was no operative death and no major complications. During the follow-up period, the child did not develop obvious symptoms, and there was no death, coronary artery complications, or other major complications.Reference Yaojun, Changwei and Rui24

Figure 9. a: Anomalous left coronary artery from the pulmonary artery with the intramural left coronary artery (1: bridging segment, 2: intramural segment); b: CT three-dimensional reconstruction image.
Coronary unroofing
It is suitable for patients with left coronary artery opening at the right lateral wall of the main pulmonary artery or the anterior wall of the right pulmonary artery, and the bridging segment and the intramural segment run perpendicular and the bridging distance is short. The ascending aorta and main pulmonary artery were dissected vertically in one step to the level of left coronary artery opening, and the intramural segment of left coronary artery was explored. Coronary unroofing was completed by direct longitudinal dissection of the main left coronary artery to the intramural segment on the side adjacent to the ascending aorta. Then a pericardial or pulmonary artery piece of appropriate size was taken and sutured continuously with the left part of the left coronary artery opening, the aortic incision, and the left coronary artery was directly isolated directly to the aortic side. See Figure 10.

Figure 10. Coronary unroofing performed in our centre. a: The intramural segment of left coronary artery (LCA) was explored. b: Unroofing of intramural coronary artery; c: A pericardial piece or pulmonary artery wall of appropriate size was taken and sutured continuously with the left part of the LCA opening and the aortic incision, and LCA was septate to the aortic side.
Coronary unroofing + coronary replantation
It is also suitable for patients with left coronary artery opening in the right lateral wall of the main pulmonary artery or the anterior wall of the right pulmonary artery. In the first step, the ascending aorta and main pulmonary artery were dissected vertically to the level of the left coronary artery opening, the intramural segment of left coronary artery was explored, and the intramural coronary artery was unroofed. The pulmonary artery wall at the upper edge of the left coronary artery opening was then made into a button and anastomosed to the corresponding position of the ascending aorta. See Figure 11.

Figure 11. Coronary unroofing and coronary reimplantation performed in our centre. a: The intramural segment of left coronary artery (LCA) was explored. b: Unroofing of intramural coronary artery; c: The wall of the pulmonary artery around the opening of LCA was made into a button and anastomosed to the corresponding position of the ascending aorta.
Coronary unroofing + left coronary artery ostium closure
It is suitable for patients with a long distance and horizontal coronary wall segment. In these patients, the bridging segment is usually short, and the proximal left coronary artery of appropriate length cannot be separated for coronary reimplantation, and the intramural segment walks horizontally, which may be behind the valve junction and cannot be unroofed in the whole process. Therefore, under the guidance of coronary probe, the location of left coronary artery leaving the aortic wall can be explored, the ascending aorta can be cut vertically to this place, and local unroofing can be performed here. The coronary intima and aortic intima of the lower half of the circumference can be intermittently sutured, and the autologous pulmonary artery piece can be taken to the coronary opening of the upper half of the circumference to expand the anastomosis and directly close the left coronary artery opening. See Figure 12.

Figure 12. Unroofing of the coronary artery + suture closure of left coronary artery (LCA) ostium. a: The intramural segment of the left coronary artery runs horizontally and at a long distance; b: The position where the left coronary artery exits the aortic wall was explored, where local unroofing was performed, and LCA ostium was closure directly.
Long-term complications
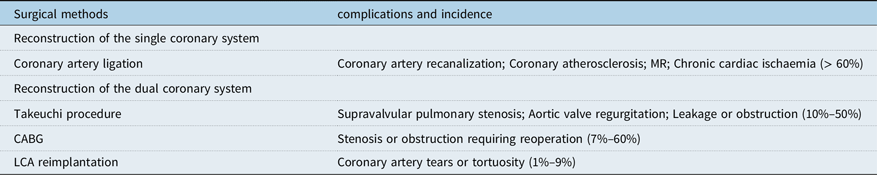
Although the complications and mortality of anomalous left coronary artery from the pulmonary artery have decreased significantly with the development of surgical techniques and postoperative assistive technology, many complications still exist in long-term postoperative follow-up, and the main complications are closely related to the choice of surgical methods, as shown in Table 2. Early coronary artery bypass grafting has been gradually abandoned due to the calcification of the graft or autologous blood vessel after long-term use, which leads to different vascular embolism. For patients with early coronary artery bypass grafting, the current treatment options include re-bypass or removal of the previous bypass vessel. The long-term complications of Takeuchi operation mainly include supravalvular pulmonary stenosis, aortic regurgitation, and baffling leakage or obstruction. Although this procedure is still used in some heart centres, it will be gradually replaced by left coronary artery reimplantation as more and more studies show the obvious disadvantages of this procedure. For patients who have undergone the above two surgical procedures in the early stage, due to the incomplete anatomical repair, the phenomenon of chronic ischaemia of the heart still exists, and some patients have severe arrhythmia during the postoperative follow-up, and some patients have sudden death. At present, most scholars believe that left coronary artery reimplantation is the best surgical plan for the treatment of anomalous left coronary artery from the pulmonary artery, and it has lower long-term complications than other surgical plans. The long-term complications of this operation mainly include coronary artery tear or tortuosity, but the strict design of the surgical plan and the use of a variety of improved surgical techniques have reduced the risk of coronary artery tear or tortuosity. Therefore, this surgical scheme is gradually accepted by more and more people.
Table 2. Long-term complications and incidence of different surgical methods
Application of mechanical assistance
Acute left heart failure is a common complication in children with severe anomalous left coronary artery from the pulmonary artery (preoperative LVEF < 30% or left ventricular end-diastolic diameter (LVEDD) > 40 mm). Mechanical assist devices can provide effective circulation to reduce the heart’s own load and promote postoperative cardiac function recovery.Reference Nasseri, Alexi-Meskishvili and Nordmeyer25 Indications for left ventricular assist device (LVAD) use include:Reference Kai, Jinghao and Wei17 ➀ Positive inotropic drug score > 20 points, BP < 55/35 mmHg and left atrial pressure > 12 mmHg; ➁ Malignant arrhythmia with pathological Q wave on electrocardiogram; ➂ LVEF < 30%; and ➃ Persistent oliguria or anuria accompanied by internal environment disorder that is difficult to correct. Extracorporeal membrane oxygenation should be used for cardiopulmonary support in children with hypoxaemia or respiratory failure. The timing of the application of mechanical assistance is also particularly important for the efficacy of mechanical assistance. Most of the children who died had long periods of circulatory instability and internal environment disorders that were difficult to correct before mechanical assistance. A multicenter retrospective study showed that the therapeutic effect of prophylactic mechanical circulatory support in the early postoperative period for children with severe anomalous left coronary artery from the pulmonary artery was significantly better than that after the occurrence of acute heart failure.Reference Weigand, Marshall, Bacha, Chen and Richmond27 According to the experience of the heart team of Shanghai Children’s Medical Center, the best time to install mechanical assistance after surgery should be within 6 hours after the end of cardiopulmonary bypass.Reference Kai, Jinghao and Wei17
Recovery of left ventricular function
Myocardial injury in children with anomalous left coronary artery from the pulmonary artery is caused by a series of pathological changes caused by myocardial ischaemia, including fibrosis of the endocardium and subendocardial papillary muscles, myocardial necrosis, and left ventricular dilatation. The selection of appropriate inotropic drugs is the key strategy for the recovery of cardiac function in children with anomalous left coronary artery from the pulmonary artery in the early postoperative period. A recent multicenter study has shown that the unique pharmacological properties of levosimendan have a strong potential to promote repair of myocardial injury in children after anomalous left coronary artery from the pulmonary artery surgery and can effectively improve cardiac function.Reference Wang, Gong and Shi26 All patients with postoperative acute left heart failure were treated with a combination of positive inotropic drugs and lyophilised recombinant human brain natriuretic peptide by the heart team of Shanghai Children’s Medical Center before 2010.Reference Kai, Jinghao and Wei17 After 2010, extracorporeal circulation devices were more likely to be used for critically ill children with LVEF<35%. Many groups of studies have shown that most children with anomalous left coronary artery from the pulmonary artery gradually recover their left ventricular function within 1 year after surgery.Reference Weigand, Marshall, Bacha, Chen and Richmond27 The mid-term follow-up results showed that the LVEF of the follow-up children was significantly higher than that before operation, and 81. 3% (91/115) of the surviving children recovered and maintained in New York Heart Association (NYHA) functional class I/ II within 1 year after operation. Older age at repair for anomalous left coronary artery from the pulmonary artery in patients with preoperative left ventricular dysfunction is the main reason for higher early mortality, and is also associated with longer time to normalisation of left ventricular function after surgery.Reference Zhang, Hu and Zhu28
Survival
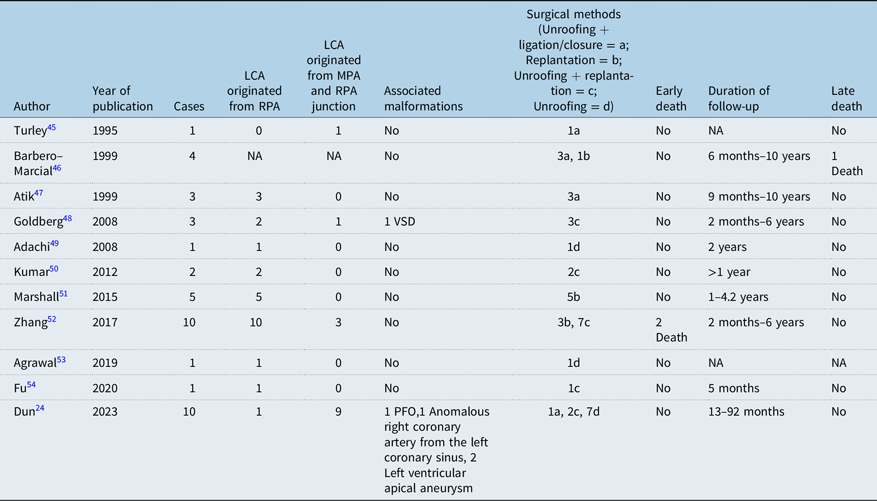
At present, Takeuchi operation and left coronary artery replantation to establish normal dual coronary artery system are mostly used in the world. However, Takeuchi’s operation has many long-term complications, such as internal pulmonary artery fistula, internal pulmonary artery obstruction, supravalvular pulmonary stenosis, and aortic valve regurgitation,Reference Pu and An31 and about one-third of patients need reoperation.Reference Martin, Mejia and Berenguel29 In recent years, it has been gradually replaced by left coronary artery reimplantation, because the latter is most in line with physiological anatomy, good postoperative effect, and few complications. The long-term coronary patency rate was high. The early mortality rate after anomalous left coronary artery from the pulmonary artery was reported to be 0–17.4%, and the late mortality rate was 0–8.6%.Reference Kumae, Aoki, Hagino, Koshiyama, Ito and Nishiori30–Reference Bhushan, Mallik, Potey, Grover, Aiyer and Jhajhria32 We also conducted a sufficient literature review and found 21 previous articles describing the mid- and long-term prognosis of follow-up after anomalous left coronary artery from the pulmonary artery repair.Reference Ma, Wang and Hua5,Reference Thomas, Chan, Alsoufi, Vinocur and Kochilas16,Reference Wang, Ding, Zhang, Zhu, Li and Li18,Reference Zhang, Hu and Zhu28,Reference Ojala, Salminen, Happonen, Pihkala, Jokinen and Sairanen33–Reference Lange, Cleuziou and Krane44 See Table 3. In addition, there were 11 previous articles describing the surgical management of anomalous left coronary artery from the pulmonary artery with intramural coronary artery.Reference Yaojun, Changwei and Rui24,Reference Turley, Szarnicki, Flachsbart, Richter, Popper and Tarnoff45–Reference Fu, Chou, Chen and Huang54 See Table 4.
Table 3. Summary of previous clinical studies on surgical treatment of anomalous left coronary artery from the pulmonary artery

NA = Not applicable.
Table 4. Summary of previous clinical studies of anomalous left coronary artery from the pulmonary artery combined with intramural left coronary artery (LCA)
Conclusion
In conclusion, no matter what type of anomalous left coronary artery from the pulmonary artery patients, theoretically once the diagnosis is confirmed, even asymptomatic patients, regardless of the collateral circulation between the left and right coronary arteries, should immediately undergo surgical treatment to promote the recovery of left ventricular function. Based on different coronary artery anatomical morphology and preoperative cardiac function, the long-term follow-up results of individualised surgical treatment of anomalous left coronary artery from the pulmonary artery children show that the prognosis is good, and the heart function of most children is significantly improved. Patients with moderate to severe mitral regurgitation should undergo mitral valvuloplasty at the same time as anomalous left coronary artery from the pulmonary artery repair. Mitral valvuloplasty can quickly improve mitral regurgitation, promote the early recovery of cardiac function after operation, and does not increase the risk of operation. Mechanical circulatory support is a safe and effective means of early postoperative transition for children with severe anomalous left coronary artery from the pulmonary artery. Anomalous left coronary artery from the pulmonary artery with intramural coronary artery is a rare anomaly. According to different anatomical types, different surgical methods can be used for anatomical correction, and the early and mid-term results are satisfactory.
Data availability statement
The data that support the findings of this study are available from the first author upon reasonable request.
Acknowledgements
We acknowledge the roles of our colleagues, perfusionists, nurses, and others involved in the care of the study participants.
Author contribution
Zhangwei Wang: Conceptualisation; Data curation; Formal analysis; Investigation; Methodology; Software; Validation; Visualisation; Writing – original draft. Kai Ma: Writing – review & editing. Shoujun Li: Writing – review & editing.
Financial support
None.
Competing interests
None.
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.



















