An estimated 40,000 children per year are diagnosed with CHD, making it the most common congenital malformation in children. Reference Hoffman and Kaplan1,Reference Reller, Strickland and Riehle-Colarusso2 In addition, approximately a quarter to one-third of children with CHD will require treatment or surgery within the first year of life. Reference Hoffman and Kaplan1,Reference Moller, Taubert and Allen3 With important medical advances, most children with CHD are now surviving through adolescence and adulthood. Reference Marelli, Ionescu-Ittu and Mackie4 Increased survival rates underscore the importance of understanding factors that affect quality of life, including understanding the risks associated with poor neurodevelopmental outcomes. Reference Morton, Ishibashi and Jonas5
Neurodevelopmental disorders are conditions characterised by deficits in functioning (e.g., cognitive, social, academic) that are apparent in early development. 6 The Diagnostic and Statistical Manual of Mental Disorders is a clinical tool that defines and categorises mental disorders and allows qualified clinicians to accurately diagnose neurodevelopmental and psychological disorders. 6 Though screening and rating scales can identify those suspected of having a disorder, diagnosis of a neurodevelopmental disorder requires more comprehensive evaluation by a qualified clinician with specialised training and expertise. Categorisation and diagnosis of neurodevelopmental disorders allows clinicians to concisely communicate a constellation of symptoms and can guide effective treatment. At times, a diagnosis is required to access certain therapeutic services. For example, applied behavioural analysis is typically a targeted intervention only available to children with a diagnosis of autism spectrum disorder. Diagnosis of a disorder also facilitates access to school and community services, such as special education services. Research to date has shown that rates of neurodevelopmental disorders, including attention-deficit/hyperactivity disorder, autism spectrum disorder (autism), and intellectual disability, are higher in children with CHD when compared to the general population. Reference Hansen, Poole and Nguyen7–Reference Feldmann, Bataillard and Ehrler22
Rates of attention-deficit/hyperactivity disorder are consistently higher in youth with CHD when compared to healthy controls; however, existing studies rarely utilise comprehensive clinical assessment. Methodologies vary but researchers typically use screening/parent report methods or focus on specific subsets of children with CHD considered to be at highest risk, resulting in variable reports of prevalence (ranging from 5 to 44%). Reference Razzaghi, Oster and Reefhuis8,Reference Holst, Kronborg and Jepsen10–Reference Holland, Cassidy and Stopp17 In studies using parent rating scales of attention-deficit/hyperactivity disorder symptoms, higher rates of attention problems are endorsed in children with CHD compared to healthy controls, Reference Holst, Kronborg and Jepsen10 with rates of attention problems in the clinically significant range for 17.8% of children with hypoplastic left heart syndrome, Reference Mahle, Clancy and Moss11 30% in complex CHD, Reference Shillingford, Glanzman and Ittenbach12 and as high as 44% in children following neonatal aortic arch repair. Reference Sistino, Atz and Simpson13 In a large national survey, which included 420 children with CHD, parents of youth with CHD were more likely to report that their child had been diagnosed with attention-deficit/hyperactivity disorder (10.3 versus 6.6%; 1.6 odds ratio). Reference Razzaghi, Oster and Reefhuis8 In a study using medical chart review of hospitalised patients aged 4–17 (1164 with CHD), more children with CHD received a diagnosis of attention-deficit/hyperactivity disorder or were prescribed stimulant medications when compared to other patients (5.1 versus 2.1%). Reference Gonzalez, Kimbro and Cutitta14 In studies using more rigorous diagnostic methods (e.g., diagnostic interviews), rates of attention-deficit/hyperactivity disorder are shown to be higher in specific CHD populations (34% in adolescents with single-ventricle CHD versus 6%; 24% in adolescents with tetralogy of Fallot versus 5%; 16% in adolescents with d-Transposition of the great arteries versus 3%). Reference DeMaso, Calderon and Taylor15–Reference Holland, Cassidy and Stopp17
Autism spectrum disorder (autism) is also frequently diagnosed in youth with CHD, though research utilising comprehensive assessment is more limited. In a large national survey, more parents of youth with CHD indicated their child had been diagnosed with autism compared to healthy controls (2.6 versus 0.6%; 4.6 odds ratio). Reference Razzaghi, Oster and Reefhuis8 In a case-control study, there was increased odds of autism diagnosis in patients with CHD (1.32 odds ratio). Reference Sigmon, Kelleman and Susi18 In a national database in Taiwan, researchers found that children with CHD were almost two times as likely to be diagnosed with autism (hazard ratio of 1.97). Reference Tsao, Lee and Jeng9 In a study of 195 4-year-olds with CHD, researchers found higher rates of autism using conservative cut-offs on rating scales (1:30.9; 3.2%), when compared to national rates (1:68; 1.47%). Reference Bean Jaworski, Flynn and Burnham19 In a clinically referred sample of 134 patients, after thorough clinical evaluation 5.9% of children with CHD were diagnosed with autism. Reference Tan, Semmel and Wolf20 More studies examining prevalence of autism in CHD utilising comprehensive evaluation are needed.
Rates of other neurodevelopmental disorders in CHD are underexplored. While meta-analysis suggests that the mean full-scale IQ of children with CHD is broadly in the average range (96.03), Reference Huisenga, La Bastide-Van Gemert and Van Bergen21,Reference Feldmann, Bataillard and Ehrler22 in a large national survey, more parents of youth with CHD indicated their child had been diagnosed with intellectual disability compared to healthy controls (5.3 versus 0.6%; 9.1 odds ratio). Reference Razzaghi, Oster and Reefhuis8 Specific learning disorders were also more prevalent in children with CHD (20.9 versus 7.6%: 3.8 odds ratio). Reference Razzaghi, Oster and Reefhuis8 Further exploration of neurodevelopmental diagnoses will be important to guide evaluations and ultimately identification of appropriate interventions.
Risk factors for poor neurodevelopmental outcomes are multi-factorial and include pre-operative factors (e.g., prematurity, antenatal diagnosis, low birth weight, genetic factors), operative factors (e.g., bypass time, neurological insults/events), cardiovascular status (e.g., oxygen saturation, oxygen intake during exercise), and social factors (e.g., socioeconomic status, parent stress); Reference Morton, Ishibashi and Jonas5,Reference Cassidy, Ilardi and Bowen23 however, the relationships between these risk factors and specific neurodevelopmental disorders in CHD are less well understood. This information is critical to develop best practice standards for assessment and care of children and adolescents with CHD.
While it is clear that children with CHD are at risk for poor neurodevelopmental outcomes, most studies limit research to a specific cardiac condition (e.g., hypoplastic left heart syndrome) or age group. Reference DeMaso, Calderon and Taylor15,Reference Tabbutt, Nord and Jarvik24 Additionally, many studies use parent report of diagnoses or parent rating scales. Reference Hansen, Poole and Nguyen7,Reference Razzaghi, Oster and Reefhuis8 In this study, we sought to characterise the rates of neurodevelopmental disorder diagnosis in a clinically referred sample of patients with any high-risk CHD (per American Heart Association and American Academy of Pediatrics [AHA/AAP] guidelines), Reference Marino, Lipkin and Newburger25 using robust diagnostic methods (e.g., neuropsychological evaluation by a licensed psychologist). Understanding the rates of these disorders in clinical samples will allow us to better understand the challenges and needs of children with CHD who are referred for evaluation as part of their routine medical care. We hypothesise that there will be higher rates of specific neurodevelopmental disorders (i.e., global developmental delay, intellectual disability, attention-deficit/hyperactivity disorder, and autism) in youth with CHD compared to the general population. We also sought to evaluate the contribution of specific medical risk factors and hypothesise that specific medical factors, such as cardiac diagnosis (single-/two-ventricle defects, aortic obstruction), diagnosed genetic disorder, history of seizure or stroke, and prematurity, will increase risk for specific diagnoses.
Materials and methods
Participants were children with CHD between the ages of 3–21 referred for neuropsychological evaluation as part of their participation in the Cardiac Neurodevelopmental Outcome Program at Children’s National. This clinical programme refers to all children who are identified as at risk for neurodevelopmental differences according to the American Heart Association and American Academy of Pediatrics Position Statement Reference Marino, Lipkin and Newburger25 for evaluation at key points in their development. These high-risk criteria include children with CHD who underwent surgical intervention in the first year of life, or if they have CHD with other clearly defined risk factors (such as extended hospitalisation, genetic disorder, and cyanosis). Data were obtained from a large prospective neuropsychological clinical research registry which records test data, diagnostic information, and basic medical and demographic information and that is approved by the Institutional Review Board at Children’s National Hospital. Caregivers are provided information about the registry and are given an opportunity to opt out of the registry at the time of the appointment. Medical charts were reviewed to confirm eligibility. Criteria for inclusion included the following: (1) age 3–21; (2) a confirmed diagnosis of CHD with high risk per American Heart Association and American Academy of Pediatrics criteria; Reference Marino, Lipkin and Newburger25 and (3) a completed neuropsychological evaluation. If a patient completed multiple neuropsychological evaluations, the most recent visit was used. The final sample included 206 patients.
Neuropsychological evaluation was performed by a licensed psychologist or trainee under the supervision of a licensed psychologist. Medical risk factors were determined based on chart review or by parent report (if records were unavailable) and included the following: prematurity (≤37 weeks of gestation), confirmed genetic condition, history of stroke, or history of seizure. Aspects of cardiac history and diagnosis were based on chart review and coded by a cardiologist (MD) according to the following: single- versus two-ventricle repair, antenatal diagnosis, and presence or absence of aortic obstruction. Aortic obstruction was defined as CHD in which flow in the aortic arch is abnormal (including hypoplastic left heart syndrome and other single ventricle with arch obstruction and two-ventricle CHD including coarctation of the aorta and interrupted aortic arch).
Data analysis was conducted with SPSS version 28. 26 Preliminary analyses examined patient demographic factors (i.e., age) and medical risk factors (i.e., prematurity, genetic condition, antenatal diagnosis, stroke, seizure, single versus two ventricle, and aortic obstruction). We computed the frequency of individuals who were diagnosed with a neurodevelopmental diagnosis (i.e., attention-deficit/hyperactivity disorder, autism, intellectual disability, specific learning disorder, communication disorder, global developmental delay, and developmental coordination disorder). We compared rates in our sample to national prevalence rates obtained from the National Health Interview Surveys from 2009–2017 for any neurodevelopmental disorder, communication disorders, attention-deficit/hyperactivity disorder, intellectual disability, autism, and specific learning disorder. Reference Zablotsky, Black and Maenner27,Reference Black, Vahratian and Hoffman28 Rates for global developmental delay and developmental coordination disorder were based on information listed in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. 6 We divided our sample into age groups for diagnoses that are limited to a specific age range (i.e., rates of global developmental delay were calculated for children ages 3–5, and intellectual disability and specific learning disorder were calculated in children ages 6 and above). We used one-sample z-tests to compare rates of neurodevelopmental diagnoses in our sample to national prevalence rates. 29 Alpha was set at .05 using the Holm adjustment for multiple comparisons. In exploratory analyses, we used chi-square analysis to determine whether medical factors (i.e., cardiac diagnosis, genetic condition, prematurity, neurologic injury) or age group (preschool [age 3–5]; school age [age 6–21]) were associated with neurodevelopmental diagnosis.
Results
Sample characteristics
Participants were aged 3–21, with a mean age of 8.6; a slight majority was male (56.8%). About one-third of the participants had single-ventricle CHD (34.0%) and one-third had aortic obstruction (33.0%). Approximately one-quarter of the sample was born premature (25.7%), and almost half received a CHD diagnosis before birth (47.1%). A genetic disorder was diagnosed in 34% of the patients in our sample. Over one-third of patients with a genetic disorder (35%) had 22q11.2 deletion, and only 8.8% of patients with a genetic disorder were diagnosed with a syndrome or deletion where intellectual disability is a core characteristic. A minority of patients have a history of stroke or seizures(17.0% stroke; 14.1% seizure). Mean IQ of this sample was in the average range (m = 90.7; SD = 20.4) and consistent with intellectual functioning described in previous research in patients with CHD. Reference Feldmann, Bataillard and Ehrler22,Reference Bellinger and Newburger30,Reference Sarrechia, Miatton and De Wolf31 See Table 1 for complete information on demographic factors and Table 2 for cardiac and medical factors in our sample. There were no differences in neurodevelopmental diagnoses based on gender or race/ethnicity.
Table 1. Demographics for clinically referred sample with CHD.
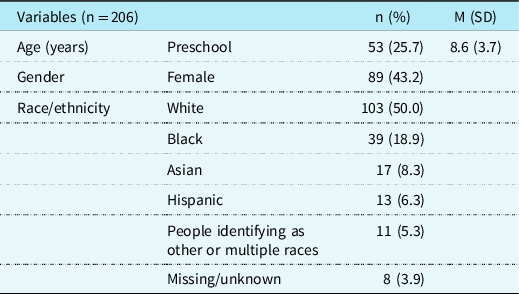
Table 2. Medical and cardiac factors for clinically referred sample with CHD.
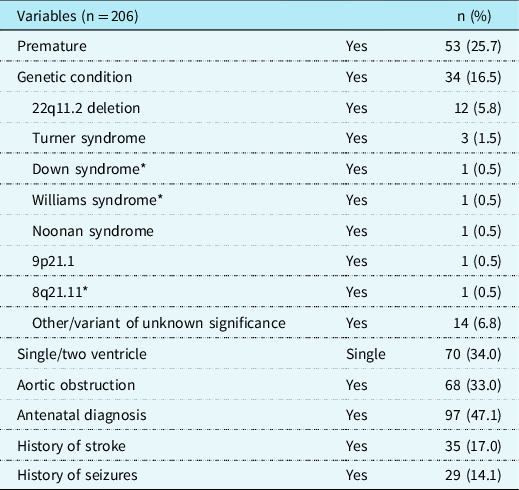
*syndromes where intellectual disability is a core characteristic
Prevalence of any neurodevelopmental diagnosis
Youth with CHD in our clinically referred sample were about five times more likely to receive a neurodevelopmental diagnosis than those in the general population (odds ratio 4.98; 95% CI 3.77–6.56). In our sample, 44% of patients had at least one neurodevelopmental diagnosis, which is significantly higher (p < .01) than the prevalence in the general population (16.93%). More than half of children aged 6 and above received a neurodevelopmental diagnosis; school-age children were about three times more likely to receive a diagnosis than preschool-age children (odds ratio 3.02; CI 1.51–6.01). No medical risk factors differentiated between children with or without any neurodevelopmental disorder. See Table 3 for relevant medical and cardiac risk factors for each diagnosis. See Table 4 for age trends for each diagnosis.
Table 3. Medical and cardiac risk factors for neurodevelopmental diagnoses for clinically referred sample with CHD.
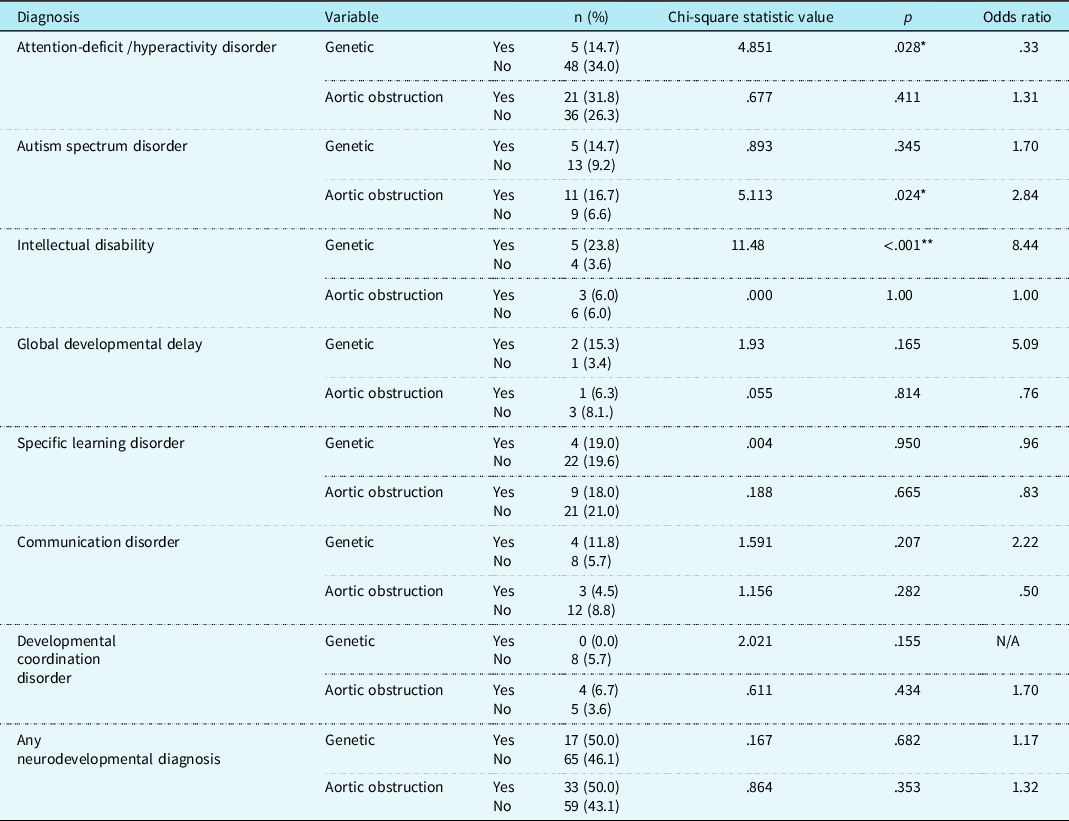
*= p < .05, **=p < .01
Table 4. Age trends for neurodevelopmental diagnoses for clinically referred sample with CHD.
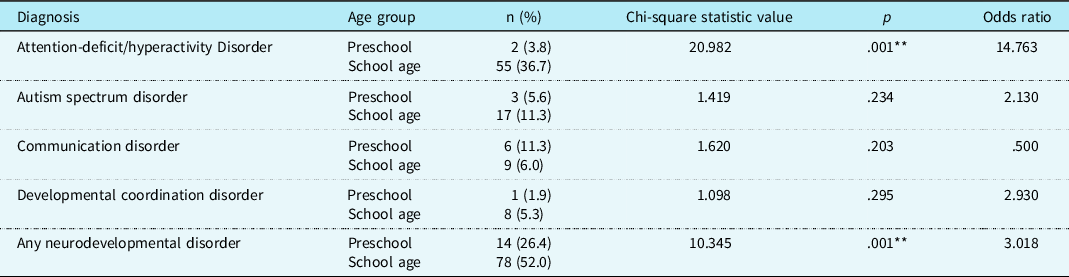
Note: Preschool= age 3–5; school age= age 6+; **=p<.01
Autism spectrum disorder
Youth with CHD in our clinically referred sample were six times more likely to be diagnosed with autism compared to those in the general population (odds ratio 6.24; 95% CI 3.92–9.93). In our sample, 9.6% of patients were diagnosed with autism, which is significantly higher (p < .01) than the prevalence in the general population (1.74%). A diagnosis of autism was more than twice as likely in children with an aortic obstruction (X 2 (1, n = 206) = 5.113, p < .05); 16.7% of patients with aortic obstruction were diagnosed with autism compared to 6.6% of patients without aortic obstruction (odds ratio 2.84; 95% CI 1.12–7.25). No other medical risk factors were associated with an autism diagnosis. Rates of autism were similar across age groups.
Attention-deficit/hyperactivity disorder
Youth with CHD in our clinically referred sample were four times more likely to receive a diagnosis of attention-deficit/hyperactivity disorder compared to those in the general population (odds ratio 4.37; 95% CI 3.21–5.93). In our sample, 27.5% of patients were diagnosed with attention-deficit/hyperactivity disorder, which is significantly higher (p < .01) than the prevalence in the general population (9.04%). Children with a genetic condition were less likely to be diagnosed with attention-deficit/hyperactivity disorder (X 2 (1, n = 175) = 4.85, p < .05); 14.7% of patients with a genetic condition were diagnosed attention-deficit/hyperactivity disorder, compared to 33.6% of patients without a genetic condition (odds ratio 0.33; 95% CI 0.12–0.92). No other medical risk factors were associated with an attention-deficit/hyperactivity disorder diagnosis. School-age children were fourteen times more likely to be diagnosed with attention-deficit/hyperactivity disorder compared to preschool-age children (X 2 (1, n = 203) = 20.98, p < .01); 36.67.7% of children ages 6 and up were diagnosed with attention-deficit/hyperactivity disorder, compared to 3.8% of children age 3–5 (odds ratio 14.76; 95% CI 3.46–63.02).
Intellectual disability
With respect to global delays, children under the age of 5 are provided with a diagnosis of global developmental delay, while a diagnosis of intellectual disability is considered for ages 6 and up (as it is challenging to assess severity of cognitive and adaptive deficits for children under age 5). 6 Thus, we divided our sample based on age group and examined rates of global developmental delay in preschoolers (age 3–5) and intellectual disability in children and adolescents (ages 6 and up). Among preschoolers, 7.5% were diagnosed with global developmental delay. In the general population, estimates for global developmental delays range from 1–3%, so it is unclear if rates are different from the general population (p < .01 using 1% estimate, p > .05 using 3% estimate). School-age children with CHD were five times more likely to be diagnosed with intellectual disability than children in the general population (odds ratio 5.53; 95% CI 2.81–10.88); 5.9% of children 6 and older in our sample were diagnosed with intellectual disability compared to 1.1% in the general population (p < .01). Children with genetic conditions were almost eight times more likely to be diagnosed with intellectual disability compared to those without a genetic condition (X 2 (1, n = 112) = 11.48, p < .01); 23.8% of patients with a genetic disorder were diagnosed with intellectual disability, compared to 3.6% of those without a genetic disorder (odds ratio 8.44; 95% CI 2.05–34.76). No other medical risk factors were associated with an intellectual disability or global developmental delay diagnosis.
Specific learning disorder
Specific learning disorders are not diagnosed until a child has received some academic instruction in a standardised way; 6 therefore, it was examined only in our school-aged patients (ages 6 and up). Among school-aged children, 19.6% of CHD patients were diagnosed with a specific learning disorder. Compared to prevalence rates reported by Zablotsky et al. (7.74%), Reference Zablotsky, Black and Maenner27 children in our sample were three times more likely to be diagnosed with a specific learning disorder (odds ratio 3.22; 95% CI 2.16–4.81). However, rates of specific learning disorder reported in the Diagnostic and Statistical Manual of Mental Disorders are quite variable, ranging from 5–15%, 6 so it is unclear if there is a significant difference relative to the general population.
Other neurodevelopmental diagnoses
Children with CHD were not more likely to be diagnosed with a communication disorder (7.2 versus 7.7% in the general population) or developmental coordination disorder (4.3 versus 5% in the general population). See Figure 1 for prevalence of all neurodevelopmental diagnoses compared to rates in the general population. Specific neurodevelopmental diagnoses were not significantly associated with other medical risk factors, including single-/two-ventricle CHD, stroke, seizure, prematurity, or antenatal diagnosis.

Figure 1 : Rates of neurodevelopmental diagnoses in a clinical sample of children with CHD compared to the general population.
Discussion
In our cohort, patients with CHD between the ages of 3 and 21 are at high risk for neurodevelopmental disorders, particularly attention-deficit/hyperactivity disorder, autism, and intellectual disability. Using more thorough clinical methods (e.g., clinical diagnostic interview and neuropsychological assessment versus parent report or survey), these findings are generally consistent with past research. We found that in our clinically referred sample, children, adolescents, and young adults with CHD were five times more likely to be diagnosed with an intellectual disability (5.9%), which is consistent with rates of intellectual disability from a national survey of patients with CHD using parent report (5.3%). Reference Razzaghi, Oster and Reefhuis8 In our clinical sample, 27% of youth with CHD were diagnosed with attention-deficit/hyperactivity disorder (odds ratio 4.37). This is higher than rates reported in a national sample of youth with CHD based on parent report (10.3%; odds ratio 1.6), Reference Razzaghi, Oster and Reefhuis8 though it is very similar to studies using more rigorous clinical methods (e.g., 17.8–44% in studies using rating scales; Reference Holst, Kronborg and Jepsen10–Reference Sistino, Atz and Simpson13 16–34% in studies using diagnostic interviews Reference DeMaso, Calderon and Taylor15–Reference Holland, Cassidy and Stopp17 ). Research generally suggests rates of autism are higher in children with CHD (2.6% based on parent report, Reference Razzaghi, Oster and Reefhuis8 3.2% based on parent ratings, Reference Bean Jaworski, Flynn and Burnham19 5.9% based on diagnostic evaluation Reference Tan, Semmel and Wolf20 ), and our results demonstrated even higher rates of autism (9.7%; odds ratio 4.37). Taken together, this pattern of results suggests more rigorous diagnostic methods (i.e., neurodevelopmental or neuropsychological evaluation) are necessary to appropriately identify children with autism or attentional disorders and supports previous assertions that regular evaluation, and not screening, is necessary for children with CHD. Reference Marino, Lipkin and Newburger25,Reference Ilardi, Sanz and Cassidy32,Reference Ware, Butcher and Latal33 Additionally, characteristics typically thought to indicate risk for worse neurodevelopmental outcomes, such as single-ventricle disease, were not associated with higher risk for neurodevelopmental diagnoses. This suggests that regardless of their specific cardiac diagnosis, all children and adolescents with high-risk CHD per American Heart Association and American Academy of Pediatrics guidelines should receive evaluation for neurodevelopmental disorders across the course of development.
Our study suggests that genetic disorders and history of aortic obstruction may be important risk factors for neurodevelopmental diagnoses associated with more substantial delays (e.g., intellectual disability and autism). Of note, many genetic disorders included in our sample are independently associated with higher risk of intellectual disability (e.g., 22q11.2 deletion), and there are a smaller number where intellectual disability is a core characteristic of the syndrome (e.g., Down syndrome). Consideration should be given to specific literature associated with genetic syndromes as appropriate, as these confer additional, specific risks. Unexpectedly, other medical factors often associated with poor outcomes, including single-ventricle CHD, prematurity, and history of seizures or stroke, were not associated with neurodevelopmental diagnoses in our study. Of note, we did not systematically collect or interpret specific neuroimaging findings in our patient group; as such, our information about neurological findings is limited. It is likely that subtler findings related to brain development, such as white matter injury or indicators of immaturities in brain development, may be more helpful in understanding these associations. However, our results remain relevant from a clinical decision-making standpoint, as this information is most often available even in lower-resourced centres. While literature suggests that prematurity is associated with developmental delay and cognitive dysfunction, its relation to specific diagnoses, including autism and attention-deficit/hyperactivity disorder, is inconclusive and dependent on other factors (including birthweight or severity of prematurity). Reference Peralta-Carcelen, Schwartz and Carcelen34 It is likely that specific medical factors contribute to specific neuropsychological outcomes in children with CHD (e.g., executive dysfunction), or more subtle dysfunction, even if they do not affect whether a child meets diagnostic criteria for a neurodevelopmental disorder. For example, patients with two-ventricle CHD, Reference Gerstle, Beebe and Drotar35 aortic obstruction and prematurity, Reference Sanz, Berl and Armour36,Reference Sanz, Wang and Berl37 have been found to have poorer executive functioning on rating scales. Additionally, Cassidy et al. found that some executive functioning skills are similar across CHD groups, while visuo-spatially mediated executive functioning skills were impaired in patients with tetralogy of Fallot and single-ventricle CHD, but within normal range for patients with transposition of the great arteries. Reference Cassidy, White and DeMaso38 Viewed in the context of this prior research, our findings suggest that patients with CHD are at risk for adverse neurodevelopmental outcomes regardless of the severity of their heart defect or other neurological risk factors. That said, the presence of a genetic disorder increases the risk for more substantial global delays (i.e., intellectual disability). Furthermore, CHD with aortic obstruction may also present increased risk to neurodevelopment, perhaps by impacting pre-natal cerebral blood flow and subsequent foetal and perinatal brain development. Reference Sanz, Wang and Berl37 For example, research suggests that children with smaller aorta diameter at birth show differences in white matter development in deeper brain regions; these types of abnormalities have been associated with neuropsychological and neurodevelopmental outcomes (e.g., cognition, motor, executive functioning). Reference Zaidi, Newburger and Wypij39,Reference von Rhein, Buchmann and Hagmann40 Additional research is needed to understand other medical and demographic risk and protective factors.
It is important to also highlight that rates of specific learning disorders were not necessarily higher in our clinical sample of children with CHD when compared to the general population, which may, on the surface, contradict prior research. That is, previous studies have shown high levels of academic service utilisation over time, with increasing rates as students get older. Reference Riehle-Colarusso, Autry and Razzaghi41 Of note, in this clinical setting, neuropsychological evaluations did not always include full academic evaluations given limitations in insurance coverage or time (though they were often completed if parents endorsed academic concerns, or data were sometimes provided by school districts); therefore, rates may represent an underestimate of specific learning disorders in this population. Regardless of whether there are differences in rates of specific learning disorder, children with CHD have higher rates of attention-deficit/hyperactivity disorder, intellectual disability, and autism, which will likely impact academic performance. Additionally, other aspects of their neuropsychological profile (e.g., attention, executive function, language) may be important factors to consider for understanding academic outcomes. In this way, we might conceptualise children with CHD being at greater risk for more broadly termed “learning disorders,” or neurodevelopmental issues that affect academic learning, as opposed to specific learning disorders such as dyslexia, dysgraphia, or dyscalculia. Reference Peterson, Boada and McGrath42
Our findings also suggest that children within different age groups present with different neurodevelopmental profiles. Specifically, preschool-age children are more likely to receive diagnoses that reflect more global impairment (e.g., global developmental delays). Problems with later developing skills, such as attention, executive functioning, and cognitive skills needed for learning, may start to become more apparent as children enter school. Given this developmental trajectory, formal diagnoses such as attention-deficit/hyperactivity disorder and specific learning disorder may not be readily detected in early childhood. This pattern is also reflected in longitudinal research that suggests children may “grow into” certain deficits, which emerge over the course of development. Reference Brosig, Bear and Allen43 This highlights the need for early monitoring for more globally impacted diagnoses. However, even if early evaluation is normal, continued evaluation over the course of development is needed as cognitive skills develop and as environmental expectations change as children grow. Furthermore, this suggests that researchers should work towards development of measures that can better predict later developmental outcomes (e.g., executive functioning screening, evaluation of phonological skills, and other predictors of academic skills) and that evaluations should go beyond formal diagnosis and include measurement of these specific skills. It is important that children with CHD continue to be evaluated for neurocognitive and neurodevelopmental problems throughout childhood and adolescence. Additionally, research suggests that up to 70% of children with CHD benefitted from neurodevelopmental evaluations (e.g., received a diagnosis, were referred for more testing, were referred for a service or therapy), which highlights the value of neurodevelopmental and neuropsychological evaluations in regard to differential diagnosis and treatment planning. Reference Glotzbach, Ward and Marietta44
It is important to note that our work examined diagnoses based on criteria in the Diagnostic and Statistical Manual of Mental Disorders. Identification of diagnoses is important, as it often leads to initiation of services and therapy and is a quick and efficient way to communicate with treatment providers in the community and schools to determine what services are needed. However, more subtle differences in functioning may be missed by looking at categorical data, and some neurodevelopmental outcomes in CHD may not be neatly captured by diagnostic criteria in the Diagnostic and Statistical Manual of Mental Disorders. Future research should look at continuous variables of neurodevelopmental outcomes and skills (e.g., neuropsychological profile, psychosocial, and quality of life measures). Looking at patterns of cognitive data and symptom report more broadly will provide better statistical power and allow for more nuanced understanding of risk and protective factors in CHD for neuropsychological factors that do not readily fit into diagnostic categories. Reference Ilardi, Sanz and Cassidy32,Reference Ware, Butcher and Latal33,Reference Sanz, Berl and Armour36
While our research contributes to understanding of neurodevelopmental outcomes in CHD, there are several study limitations that should be considered. First, our work was cross-sectional; additional longitudinal research is needed to understand possible developmental cascades and improve prediction of later outcomes. Additionally, our study was a retrospective chart review and included patients who were referred for and attended a neuropsychological evaluation. Though all families who meet high-risk criteria are referred for participation in neurodevelopmental follow-up, parents who had more concerns about their child’s neuropsychological functioning may have been more likely to attend visits, which may lead to an overestimate of diagnoses. Despite this, our prevalence rates were remarkably similar to those presented in community-based research using surveys or other less sensitive techniques and likely reflect rates that are seen in clinically referred samples. Furthermore, prevalence rates were compared to the National Health Interview Survey data. Reference Zablotsky, Black and Maenner27 This comparison was selected due to its large sample size, inclusion of a similar age range of patients, and inclusiveness of several similar neurodevelopmental diagnoses. However, limitations of this comparison include its reliance on parent report of diagnoses, which may underestimate the prevalence of some disorders requiring more comprehensive assessment. Fortunately, prevalence rates from Zablotsky et al. are generally consistent with prevalence rates reported in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. 6 Our research also evaluated data from one outpatient centre, which may limit the generalisability of findings; multi-site or multi-centre studies and data registries will allow us to improve generalisability and statistical power. These larger samples will also allow researchers to more thoroughly examine specific medical factors and broader neuropsychological profiles.
There were also limitations with medical risk factors. Our work did not include several important risk factors, including length of hospital stay or perioperative risk factors, and sample sizes for specific risk groups might not have been large enough to detect differences. Additionally, while perioperative MRI is currently standard of care, MRIs may not have been completed for some patients, particularly older teens, which may underestimate other neurological abnormalities (such as white matter injuries, immaturity, encephalomalacia, periventricular leukomalacia) beyond overt stroke or seizures. Using a wider variety of neuroimaging approaches (such as electroencephalogram [EEG], diffusion tensor imaging [DTI], MRI) will also help us better understand neurologic risk factors and merits investigation in future studies. More work is needed to better operationalise other medical risk factors, which will also require larger sample sizes. Furthermore, other factors, such as socioeconomic status, distance from hospital, or other barriers to care, including insurance, may limit access to evaluations and representation in clinical research. Future research should examine sociodemographic factors that may contribute to health disparities and neurodevelopmental outcomes. It will also be important for future research to examine modifiable risk and protective factors for neurodevelopmental outcomes, including access to early intervention services and family factors (e.g., parent stress).
Despite these limitations, our work has several clinical implications. First, clinicians completing neurodevelopmental evaluations should consider that children, adolescents, and young adults with CHD are at risk for several neurodevelopmental disorders, including autism, attention-deficit/hyperactivity disorder, and intellectual disability, and therefore, comprehensive evaluations should assess social cognition, attention and executive functioning, overall cognitive functioning, and adaptive skills. Additionally, clinicians should recognise that some diagnoses, such as attention-deficit/hyperactivity disorder and specific learning disorder, may not become apparent until later elementary school, when there is an increase in the workload, more expectations for independence, and higher demands for learning and executive functioning (e.g., focused attention, organisation, and planning). In addition, children, adolescents, and young adults with CHD are at high risk for neurodevelopmental disorders and as such should be referred for evaluation over the course of development if they meet American Heart Association and American Academy of Pediatrics high-risk criteria, Reference Marino, Lipkin and Newburger25 regardless of specific cardiac diagnosis or medical risk factors. If psychologists and/or neuropsychologists are not readily available, clinicians are encouraged to partner with community agencies, such as early intervention programmes and schools, and with ancillary service providers (such as speech-language, occupational, and physical therapists), which can help provide some evaluation that will help identify children at risk and connect them with services, even in centres with fewer resources.
Neuropsychological evaluations are an important aspect of cardiac care. Our research demonstrates the importance of monitoring neurodevelopmental outcomes. Additionally, it is important for all children and adolescents to be assessed, regardless of the severity of their CHD, though children with aortic obstruction or genetic disorders may have greater risk for neurodevelopmental disorders. Additionally, children should continue to be monitored over time, even if early evaluation is normal, as some diagnoses may not be apparent until later in development. Data registries and multi-centre studies will improve our ability to understand risk factors for poor outcomes. Work that examines resiliency and protective factors will also allow for better models of care and targets for intervention.
Acknowledgements
Thank you to the patients, families, clinicians, and staff of the Cardiac Neurodevelopmental Outcome Program at Children’s National Hospital.
Financial support
This work was supported by the District of Columbia Intellectual and Developmental Disabilities Research Center (DC-IDDRC) Award U54HD090257 by NICHD (PI: V. Gallo).
Conflicts of interest
None








