Arterial switch operation for transposition of the great arteries has been performed with excellent outcomes. Reference Vida, Zanotto and Zanotto1 Our experiences showed very satisfactory outcomes, and only two among 77 arterial switch operations required surgical intervention for asymptomatic coronary arterial stenosis during long-term follow-up. Reference Shim, Jun and Yang2 Despite the scarcity of coronary insufficiency after arterial switch operation, Choi et al. reported a successful coronary artery bypass graft in a 3-month-old male. Reference Choi, Pyo, Choi and Chung3 Though rare, percutaneous coronary intervention in children has been performed. Not only small vessel size, but also anomalous coronary artery anatomy contributes to the complexity of percutaneous coronary intervention, especially in infants who required previous correction of congenital heart disease. Successful percutaneous coronary intervention in seven children with transposition of the great arteries or double outlet of the right ventricle, as well as a case of percutaneous coronary intervention in an 8-month-old infant after correction of anomalous left coronary artery from pulmonary artery, were reported. Reference Callahan, Lock, Shah and Marshall4,Reference Cai, Kramer, Bandisode and Fernandes5 Due to the rarity of these experiences, we report a complicated case with serious coronary insufficiency after arterial switch operation and subsequent successful percutaneous coronary intervention in an infant.
Case
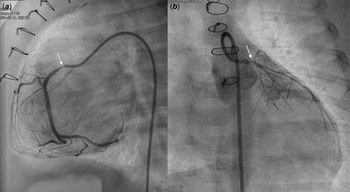
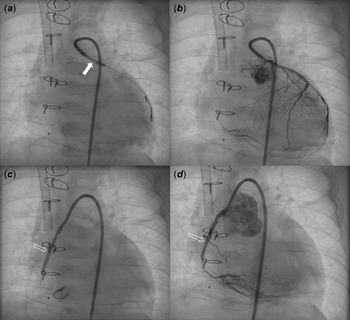
A male patient with d-transposition of the great arteries with an intact ventricular septum underwent arterial switch operation at 7 days of age. He had abnormal coronary arteries, both arising from a single coronary sinus, and an unusual left coronary artery with an intramural course. Both coronary arteries were re-implanted successfully by unroofing the left coronary artery ostium. One month after arterial switch operation, he presented to the emergency department with severe left ventricular dysfunction (left ventricle ejection fraction = 20%) and heart failure. Urgent coronary angiography confirmed severe stenosis of the proximal right coronary artery (Fig 1a) and near total occlusion of the left coronary artery ostium (Fig 1b). Emergent angioplasty of the left coronary artery ostium was performed in the 5-kg-body-weight patient. After post-operative recovery and improvement of left ventricular function, ventricular dysfunction (ejection fraction = 15%) re-developed at 4 months of age, approximately 2 months after the second operation. Percutaneous coronary intervention was planned in the infant, now 7 kg of body weight, to be performed by a paediatric cardiologist and adult interventional cardiologist. Elective extracorporeal membrane oxygenation of the right internal jugular vein-right common carotid artery was performed before percutaneous coronary intervention. Coronary angiography showed 95% segmental stenosis of the left coronary artery and 99% diffuse stenosis of the right coronary artery. Left coronary artery angioplasty with a 2.0 × 15-mm Maverick balloon was conducted successfully at 8 atm (Fig 2a), and 30% residual stenosis without dissection was confirmed (Fig 2b). Due to the unusual position of the right coronary artery, a 5-Fr JR 4.0 guiding catheter was reshaped and engaged properly with the right coronary artery. Runthrough and Sion guidewires were used to cross the lesion. Because 50% residual stenosis was found in the right coronary artery after angioplasty with 2.0 × 15-mm Maverick balloon at 12 atm, stent implantation with a 2.25 × 26-mm Orsiro stent at 12 atm was performed successfully (Fig 2c). After post-adjunctive balloon dilatation with 2.5 × 8-mm NC balloon, 10% residual stenosis in the right coronary artery was confirmed without dissection (Fig 2d). The total fluoroscopy time was 85 minutes. The patient was weaned successfully from extracorporeal membrane oxygenation the next day. Echocardiography at 7 days after percutaneous coronary intervention showed recovered ventricular contraction (left ventricle ejection fraction = 60%) but dilated left ventricle. The patient’s symptoms were relieved, and he was discharged on dual antiplatelet therapy with aspirin and clopidogrel. No complications related to femoral arterial cannulation in such a small patient were detected.
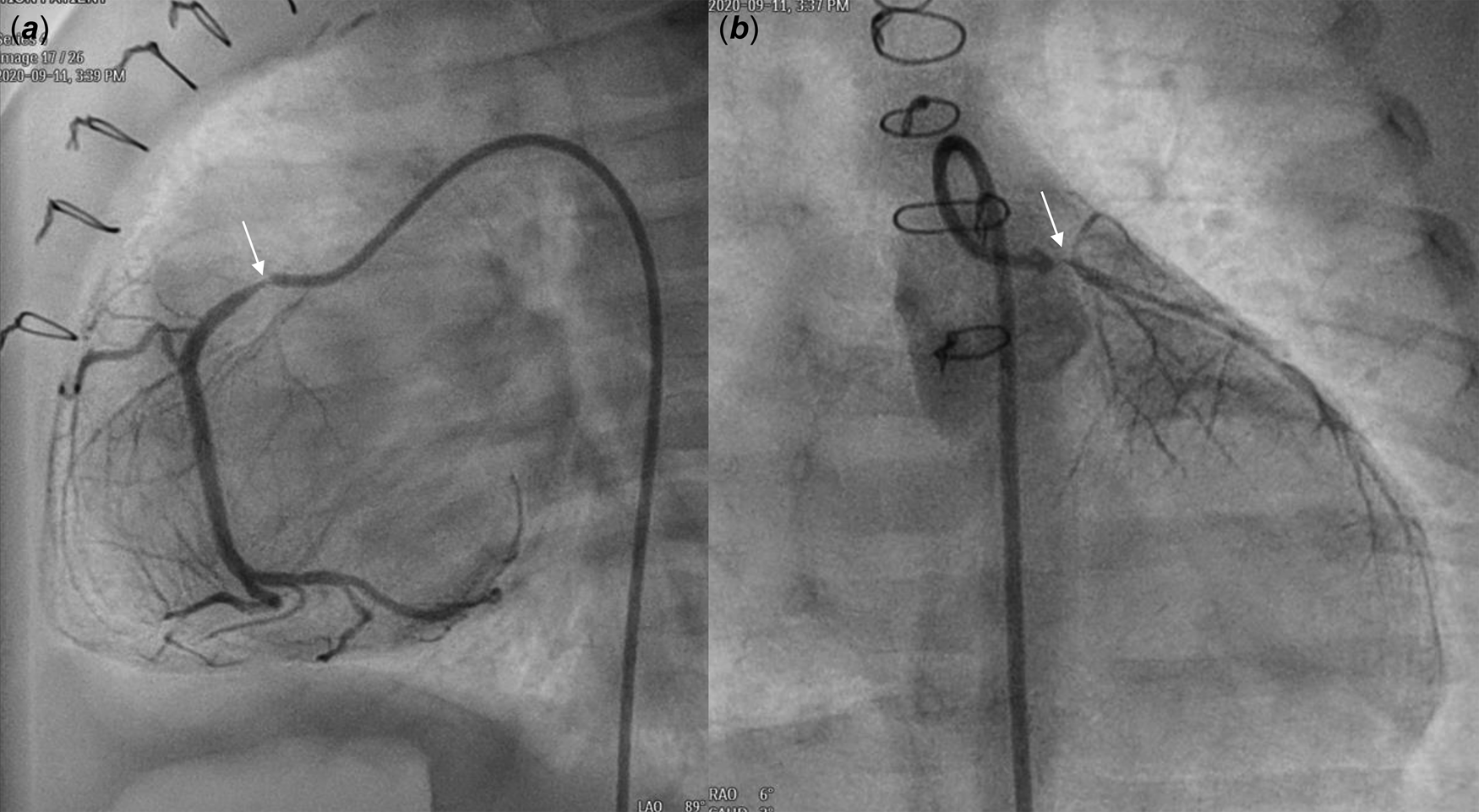
Figure 1. Angiography at one month after ASO revealed severe stenosis (thin arrow) of the proximal RCA ( a ) and nearly total occlusion (thin arrow) of the LCA ostium ( b ). ASO: arterial switch operation, RCA: right coronary artery, LCA: left coronary artery.

Figure 2. Coronary angiography after PCI showed balloon angioplasty (thick arrow) for LCA stenosis ( a ) with mild residual stenosis ( b ) and stent implantation (blank arrow) for RCA severe stenosis ( c ) with minimal residual stenosis ( d ). PCI: percutaneous coronary intervention, LCA: left coronary artery, RCA: right coronary artery.
Discussion
This patient showed definite symptoms and marked ventricular dysfunction due to severe coronary stenosis early after arterial switch operation. Coronary intervention should be attempted in patients with ischemic symptoms and signs, even in the early period after arterial switch operation. The most common reason for coronary complications after arterial switch operation is an intramural course of the coronary artery, as in our patient. There can be several reasons for stenosis, including thrombus formation, intimal proliferation, excessive scar formation or distortion, and compression. We did not perform coronary artery bypass graft at the onset of ischemic symptoms due to technical difficulties related to the small size of the internal mammary artery. Although previous reports have shown satisfactory outcomes after percutaneous coronary intervention in small children after arterial switch operation, Reference Kampmann, Kuroczynski, Trubel, Knuf, Schneider and Heinemann6-Reference Hausdorf, Kampmann and Schneider8 it is a very risky procedure with various peri-procedural complications. Reference Schneider, Wiebe, Hraska and Zartner9 Pre-procedural extracorporeal membrane oxygenation application might be very helpful in a symptomatic and unstable infant. From Callahan’s experience, successful percutaneous coronary interventions were performed on extracorporeal membrane oxygenation support in 46% of patients. Reference Callahan, Lock, Shah and Marshall4 One of the trickiest procedures was catheter engagement in the implanted coronary artery. Reshaping of the guiding catheter often was helpful. Reference Cai, Kramer, Bandisode and Fernandes5 Percutaneous coronary intervention in these patients should be performed carefully to prevent dissection due to the small size of vessels. In addition, a somewhat larger balloon and stent were required. The location, length, and mechanism of stenosis should be established for successful procedures. Over-expansion was recommended to avoid restenosis. Reference Kampmann, Kuroczynski, Trubel, Knuf, Schneider and Heinemann6 The long-term outcomes of percutaneous coronary intervention in patients after arterial switch operation remain unclear because of the small number of patients. From percutaneous coronary intervention data across all indications in children, at least one re-dilatation in 73% of implanted stents and stent thrombosis in up to 3% of children were reported. Reference Stanfill, Nykanen, Osorio, Whalen, Burke and Zahn10,Reference Shaffer, Mullins and Grifka11 Nevertheless, standard antiplatelet therapy regimens and duration have not been established, though dual antiplatelet therapy for 6 months is tolerated in general. For this patient, we are planning to use aspirin and clopidogrel for 6 months, and coronary angiography will be repeated. In the long term, we are considering right coronary bypass graft surgery.
Conclusion
Percutaneous coronary intervention in infants remains a challenging procedure. Balloon dilatation and stent implantation are feasible in infants who develop coronary obstruction and acute heart dysfunction after coronary re-implantation with arterial switch operation for transposition of the great arteries. Extracorporeal membrane oxygenation support during percutaneous coronary intervention is crucial for infants exhibiting symptomatic heart failure.
Acknowledgement
None.
Financial support
This research received no specific grant from any funding agency, in the commercial or not-for-profit sector.
Conflict of interest
None.
Ethical standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees (Samsung medical center).