A patent ductus arteriosus in preterm infants is frequently associated with severe cardiopulmonary complications, such as left ventricular volume overload, pulmonary oedema, and impairment of lung compliance.Reference Sallmon, Koehne and Hansmann1–Reference Vrancken, van Heijst and de Boode3 Thus, understanding the mechanisms that control ductal closure is pivotal in order to provide tailored care to at-risk neonates.Reference Gillam-Krakauer, Hagadorn and Reese4 Previously, Echtler et al. reported that platelets are recruited to the ductal endothelium after birth and contribute to ductal closure and subsequent vascular remodelling.Reference Echtler, Stark and Lorenz5 However, the clinical significance of these animal experiments is still controversial. Low platelet counts are frequently observed among preterm infants, but several studies on their role in spontaneous or pharmacological ductus arteriosus closure produced ambiguous or conflicting results.Reference Cremer, Sallmon, Kling, Buhrer and Dame6–Reference Mitra, Chan and Paes8 We previously reported that lower platelet counts on the first postnatal day were not associated with a higher incidence of patent ductus arteriosus in 1430 very low birth weight infants.Reference Sallmon, Weber and Huning9 However, in infants who underwent pharmacological treatment with cyclooxygenase inhibitors (ibuprofen/indomethacin) for haemodynamically significant patent ductus arteriosus, low platelet counts during the first postnatal week contributed to an increased rate of treatment failure.Reference Sallmon, Weber and Dirks10 Collectively, these results suggest that the development of thrombocytopenia during the first week of postnatal life contributes to failure of ductus arteriosus closure.
The immature platelet fraction is a novel innovative tool to examine platelet production from peripheral blood samples.Reference Cremer, Sallmon, Kling, Buhrer and Dame6 Immature platelet fraction values have been shown to predict the course of thrombocytopenia in preterm neonates and thus may prove useful in guiding platelet transfusion management.Reference Cremer, Paetzold and Schmalisch11,Reference Cremer, Weimann, Schmalisch, Hammer, Buhrer and Dame12 Furthermore, immature platelets have distinct functional features that might affect haemostasis independently from total platelet counts.Reference Lador, Leshem-Lev, Spectre, Abelow, Kornowski and Lev13 To date, the proportion of mature and immature platelet counts and their possible association with haemodynamically significant patent ductus arteriosus have not been evaluated in preterm neonates. In this observational study, we investigated the number of mature and immature platelets during the first week of postnatal life and their possible association with the incidence of haemodynamically significant patent ductus arteriosus in very low birth weight preterm infants.
Methods
Patients, blood parameters, and clinical data
The study was conducted at the neonatal ICU, Department of Neonatology, Charité University Medical Center, Berlin, Germany (2009–2012). Blood sampling was performed at day 1, day 3 (±1 day), and day 7 (±2 days) after birth. Blood count parameters were measured using a Sysmex XE-2100 (Sysmex, Kobe, Japan) blood analyser as previously described.Reference Cremer, Paetzold and Schmalisch11,Reference Cremer, Weimann, Schmalisch, Hammer, Buhrer and Dame12 Immature platelet values are reported as absolute values per nanolitre (immature platelet count) and as percentage (immature platelet fraction). Mature platelet counts were obtained by using the following formula: mature platelet count = total platelet count – immature platelet count. The infants were grouped according to patent ductus arteriosus status (echocardiography on day 7, see below). Clinical data were obtained by chart reviews. All very low birth weight infants (birth weight < 1500 g) admitted to the unit were eligible if no cardiac malformation or other genetic or syndromic disorders were present. Parental written consent for blood sampling and data collection was obtained after patient admission to the neonatal ICU. The study has been approved by the local ethics committee of Charité – Universitätsmedizin Berlin, Germany (EA1/229/08).
Diagnosis of haemodynamically significant patent ductus arteriosus
Infants were examined for a haemodynamically significant patent ductus arteriosus on postnatal day 7 (±2 days) as previously described.Reference Sallmon, Weber and Huning9,Reference Groves, Singh and Dempsey14,Reference van Laere, van Overmeire and Gupta15 Echocardiographic evaluation included shunt direction of a patent ductus arteriosus in colour Doppler mode (high upper parasternal short axis) as well as the minimal internal ductal diameter detected within three to five measurements taken in B-mode. The left atrium to aortic root ratio was measured by M-mode (parasternal long axis). Doppler measurement of the resistance index (anterior cerebral artery) was performed at the same time. A patent ductus arteriosus with left-to-right shunt was considered haemodynamically significant if a respiratory set back with a supplemental oxygen requirement > 30% and/or mechanical ventilation, a left atrium to aortic root ratio ≥ 1.4 in the echocardiogram, ductal diameter ≥ 2.5 mm, and/or a decreased end-diastolic flow in the anterior cerebral artery with a resistance index ≥ 0.85 in the cerebral ultrasound were present. There has been no change in the clinical and echocardiographic criteria used to determine haemodynamic significance of a patent ductus arteriosus during the study period.
Statistics
Values are expressed as absolute number of patients or percentage values unless stated otherwise. Demographic characteristics are given as median and range. Comparisons between groups were made by Kruskal–Wallis or Mann Whitney U tests as appropriate for continuously scaled data and by chi-square test for categorical data. p-Values < 0.05 were considered statistically significant. Statistical analyses, including receiver operating characteristic curve analysis and linear regression analysis, were carried out using IBM SPSS Statistics 22 software.
Results
Clinical data and incidence of haemodynamically significant patent ductus arteriosus
A total of 69 very low birth weight infants were included in the study. Of 69 infants, 24 were diagnosed with a haemodynamically significant patent ductus arteriosus (34.8%). Infants with haemodynamically significant patent ductus arteriosus were more immature at birth (25 0/7 versus 29 2/7, p < 0.01) and exhibited a lower birth weight (mean 1108 g versus 702 g, p < 0.01) while gender distribution did not differ significantly between groups (Table 1).
Table 1. Demographic characteristics of the study population
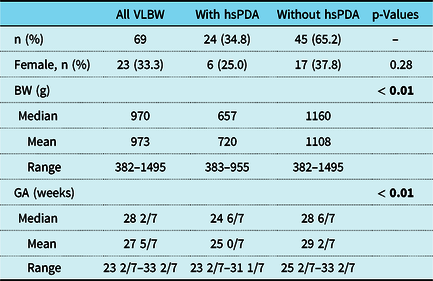
BW = birth weight; GA = gestational age; hsPDA = haemodynamically significant patent ductus arteriosus; VLBW = very low birth weight.
Mature and immature platelets during the first week of life
The mature platelet counts on postnatal day 1 did not differ significantly between infants with and without haemodynamically significant patent ductus arteriosus (p = 0.068). However, mature platelet counts were significantly lower in infants with haemodynamically significant patent ductus arteriosus on days 3 and 7 of life (p = 0.036 and <0.01, respectively, Fig 1). Infants with and without haemodynamically significant patent ductus arteriosus did not show any significant differences in immature platelet fraction (percentage) values on days 1 and 3 of life (p = 0.46 and 0.07, respectively), while infants with haemodynamically significant patent ductus arteriosus exhibited higher immature platelet fraction (percentage) values on postnatal day 7 (p < 0.01). Of note, the absolute immature platelet counts were similar between groups at all time points (Fig 1).
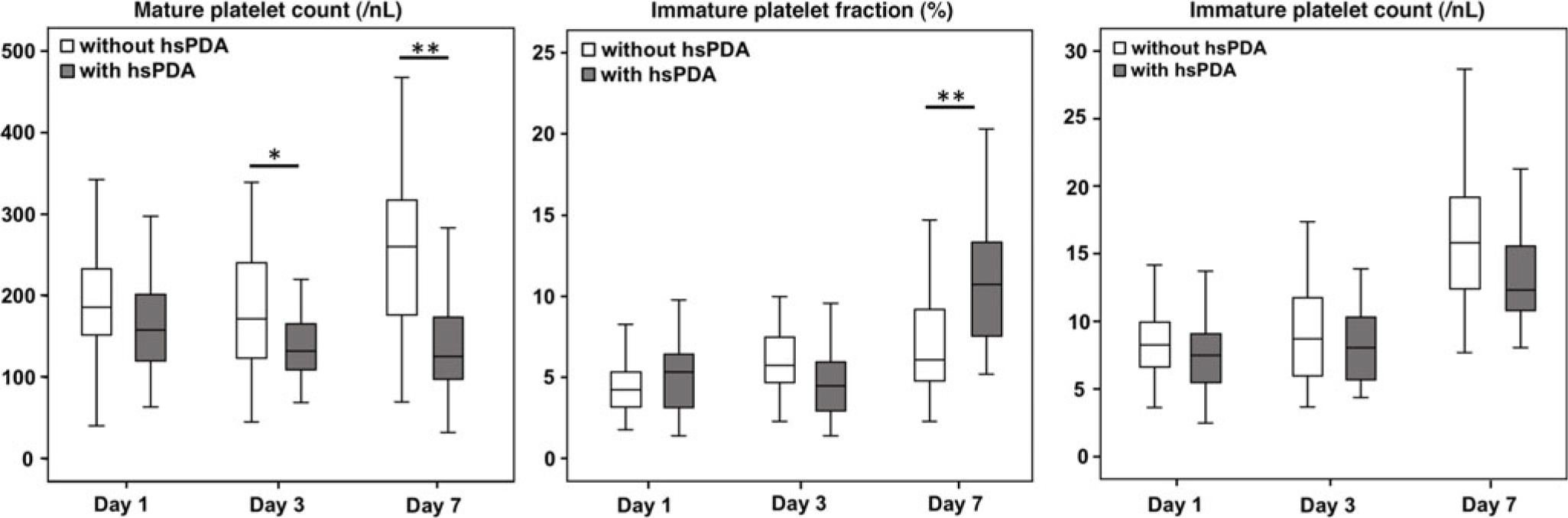
Figure 1. Boxplot: Mature platelet count and immature platelets (percentage and absolute count) in very low birth weight infants with and without haemodynamically significant patent ductus arteriosus. The boxplots display median, and 25th and 75th percentiles among the different groups. hsPDA = haemodynamically significant patent ductus arteriosus. Mature platelet and immature platelet counts are given per nanolitre. * p < 0.05, ** p < 0.01.
Receiver operating characteristic curve analysis (platelet count, immature platelet fraction, gestational age, and birth weight)
Receiver operating characteristic curve analyses were performed in order to further characterise the relationship between mature and immature platelets, birth weight, gestational age, and haemodynamically significant patent ductus arteriosus (Table 2; Fig 2). Significant associations were found for gestational age, birth weight, and mature platelet counts on days 3 and 7, which all showed an area under the curve > 0.5, indicating a negative correlation between these variables and the incidence of a haemodynamically significant patent ductus arteriosus. Immature platelet fraction (percentage) values on days 3 and 7 of life exhibited an area under the curve < 0.5 and, thus, a positive correlation with haemodynamically significant patent ductus arteriosus (Table 2; Fig 2).
Table 2. ROC curve analyses in VLBW and ELBW infants
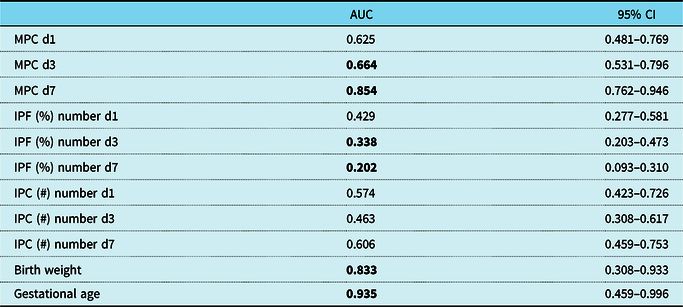
Statistical significance is indicated by bold values.
AUC = area under the curve; CI = confidence interval; d = day of life; ELBW = extremely low birth weight; IPC (#) = immature platelet count; IPF (%) = immature platelet fraction (percentage); MPC = mature platelet count; ROC = receiver operating characteristic; VLBW = very low birth weight.
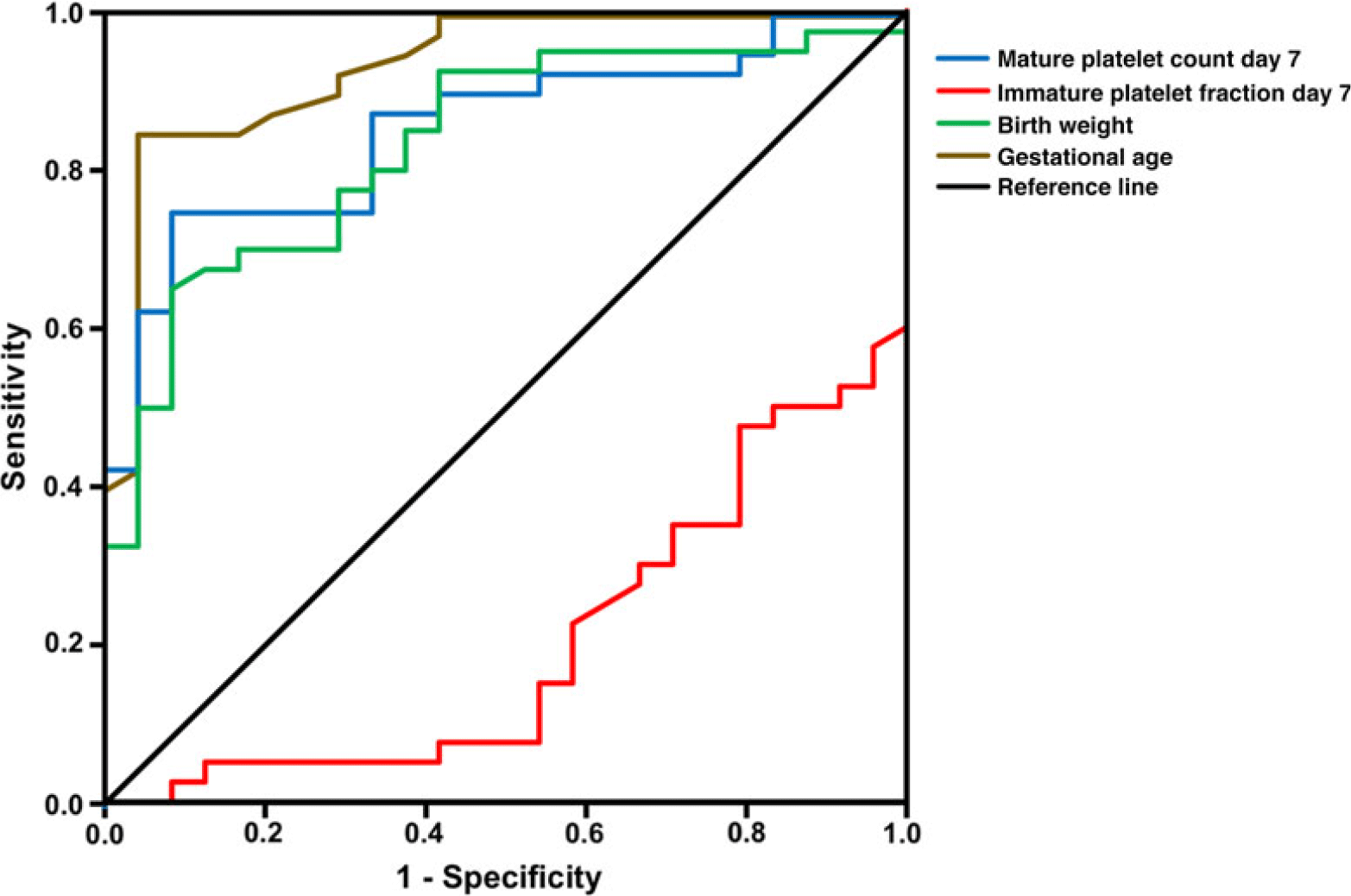
Figure 2. Receiver operating characteristic (ROC) curve analyses. ROC curve analysis for mature platelet counts and immature platelet fraction (percentage) on day 7 of life, birth weight, and gestational age comparing infants with and without haemodynamically significant patent ductus arteriosus (hsPDA). All ROC curves presented in this figure show an unequal division resulting in an area under the curve (AUC) of > 0.5 or < 0.5 (for confidence intervals, see Table 2). An AUC > 0.5 (gestational age, birth weight, and mature platelet day 7) indicates a negative and an AUC < 0.5 (IPF day 7) a positive correlation between the variable and hsPDA incidence. IPF = immature platelet fraction (percentage); MPC = mature platelet count.
Logistic regression analysis
In addition, logistic regression analysis was performed to investigate whether mature platelet counts and immature platelet fraction values on postnatal day 7 are independent risk factors for haemodynamically significant patent ductus arteriosus. While gestational age, birth weight, and mature platelet counts on day 7 were identified as independent risk factors for haemodynamically significant patent ductus arteriosus, immature platelet fraction (percentage) on postnatal day 7 was not found to be an independent predictor of haemodynamically significant patent ductus arteriosus (p = 0.585; Table 3).
Table 3. Logistic regression analysis for prediction of haemodynamically significant patent ductus arteriosus
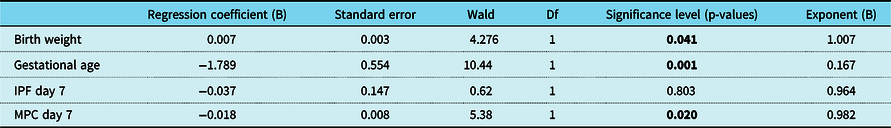
66/69 (95.7%) of cases with a complete dataset were included in this analysis. Statistical significance is indicated by bold values.
Df = degree of freedom; IPF = immature platelet fraction (percentage); MPC = mature platelet count; Wald = Wald chi-square test.
Discussion
The role of platelets in ductal closure has first been demonstrated in murine models.Reference Echtler, Stark and Lorenz5 Observational studies on the clinical significance of this finding in preterm infants have yielded conflicting results. However, two recent meta-analyses indicate a moderate association between low platelet counts in preterm infants and failure of spontaneous and pharmacologically induced ductus arteriosus closure.Reference Simon, van Zogchel, Bas-Suarez, Cavallaro, Clyman and Villamor7,Reference Mitra, Chan and Paes8 We previously demonstrated in 1430 very low birth weight infants that low platelet counts within the first 24 hours after birth were not associated with patent ductus arteriosus, while low platelet counts before and during cyclooxygenase inhibitor treatment were moderately associated with pharmacological ductus arteriosus treatment failure.Reference Sallmon, Weber and Huning9,Reference Sallmon, Weber and Dirks10 Interestingly, a recent randomised controlled trial could not demonstrate any positive effect of platelet transfusions on patent ductus arteriosus in preterm infants.Reference Kumar, Dutta, Sundaram, Saini, Sharma and Varma16 We and others therefore hypothesised that impaired platelet function, rather than platelet number, contributes to spontaneous or pharmacologically induced ductus arteriosus closure in preterm infants.Reference Sallmon, Weber, von Gise, Koehne and Hansmann17–Reference Sallmon, Barikbin, Koehne, von Gise and Hansmann19 Two recent studies support this hypothesis: Collagen-adenosine-diphosphate closure times > 130 seconds (platelet function analyser) were independently associated with ductal patency.Reference Kahvecioglu, Erdeve and Akduman20 Moreover, impaired platelet function due to inflammation may contribute to failure of ductal closure in preterm infants with infection.Reference Meinarde, Hillman, Rizzotti, Basquiera, Tabares and Cuestas21
The immature platelet fraction is a modern laboratory parameter that assesses the number of newly released platelets from peripheral blood samples. Immature platelet fraction measurements have been reported in preterm neonates and may prove useful in guiding platelet transfusion therapy.Reference Cremer, Paetzold and Schmalisch11,Reference Cremer, Weimann, Schmalisch, Hammer, Buhrer and Dame12,Reference MacQueen, Christensen and Henry22 Similar to the reticulocyte count in anaemia, the immature platelet fraction increases in response to thrombocytopenia, if megakaryopoiesis and thrombopoiesis are intact.Reference Cremer, Sallmon, Kling, Buhrer and Dame6 A recent study found an association between immature platelet fraction (percentage) and bleeding risk in children with immune thrombocytopenic purpura, independently from platelet counts.Reference McDonnell, Bride, Lim, Paessler, Witmer and Lambert23 The latter results support previous findings on immature platelets having distinct functional properties which affect their haemostatic potential.Reference Lador, Leshem-Lev, Spectre, Abelow, Kornowski and Lev13
Based on these observations, we hypothesised that, due to their distinct functional properties, mature and immature platelets are independently associated with incidence of haemodynamically significant ductus arteriosus in preterm infants. Here, for the first time, we show that lower absolute counts of mature platelets on days 3 and 7, but not on day 1 after birth, are associated with ductal patency.Reference Sallmon, Weber and Huning9,Reference Sallmon, Weber and Dirks10
In addition, we found higher immature platelet fraction (percentage) values in infants with haemodynamically significant patent ductus arteriosus as compared to those without. However, logistic regression analysis revealed that only mature platelet counts on postnatal day 7, in contrast to immature platelet fraction values, were independently associated with haemodynamically significant patent ductus arteriosus in our cohort. Our results suggest that lower counts of mature platelet contribute to a higher incidence of haemodynamically significant patent ductus arteriosus. In contrast, immature platelet fraction values, which can be regarded as a physiological response to thrombocytopenia in preterm infants, do not independently affect ductal closure. Of note, we found similar results indicating a role for lower mature platelet counts in failed spontaneous ductal closure when comparing all infants with patent ductus arteriosus (not only haemodynamically significant ones) to those without a patent ductus arteriosus (data not shown).
Our study is limited by its relatively low sample size. Thus, we were unable to assess a potential association between low mature platelet counts and treatment failure as only 24 infants underwent pharmacological treatment for patent ductus arteriosus in our cohort. In addition, the current study did not address a potential association between inflammation, as seen in septic preterm neonates, mature and immature platelets, and ductus arteriosus. Furthermore, ductal closure is a process that is characterised by a complex interplay between molecular and morphological changes within the vessel wall, which, in addition to platelets, is triggered by vascular adhesion and migration of mononuclear blood cells to the muscular layer of the vessel wall.Reference Waleh, Seidner and McCurnin24 Assessing those complex molecular and cellular processes requires a highly sophisticated experimental design and is beyond the scope of this paper. Thus, further research is required to elucidate the complex interplay between immature and mature platelets on ductal closure and to assess their specific functions and association with bleeding risk and thromboembolic complications in preterm infants.Reference Ibrahim, Schutt, Hannawi, DeLao, Barker and Kleiman25,Reference Matter, Ragab, Roushdy, Ahmed, Aly and Ismail26
Acknowledgement
We thank Silke Wilitzki for her expert assistance.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
Malte Cremer served as an advisory board member for Sysmex Deutschland GmbH, Hamburg, Germany.
Ethical Standards
The study was carried out in accordance with national and international recommendations, including the Declaration of Helsinki, and has been approved by the local ethics committee (EA1/229/08). Written informed parental consent was obtained.







