The optimal model to deliver congenital cardiac surgical care remains elusive and a subject of debate. The many institutions in the United States of America offering such care are heterogeneous in size, outcomes, resources, and structure with significant turnover of some programmes. As such, there is still room for further optimisation of such highly specialised surgical care. The case volume–outcome relationship, as well as the nuance of complex disease, is well described and continues to be a centrepiece of the case for regionalisation. Reference Jenkins, Newburger, Lock, Davis, Coffman and Iezzoni1–Reference Oster, Strickland and Mahle6 However, as social determinants of health have been described more thoroughly, it is apparent that regionalisation could exacerbate healthcare disparities along socio-economic lines leaving the most vulnerable, and some groups already disproportionally affected by congenital heart disease, with even less access to essential care. Reference Davey, Sinha, Lee, Gauthier and Flores7–Reference Welke, Pasquali and Lin11
In 2010, we proposed that joint programmes, consisting of two or more programmes partnering and collaborating on the delivery of cardiac care could provide good outcomes without compromising geographic accessibility. Reference DeCampli12 International success with joint programmes as a means of extending complex care to geographically isolated areas had been previously reported. Reference Iacona, Giamberti and Abella13 In our prior survey study of paediatric cardiac programmes in the United States of America, we reported characteristics of joint programmes and described potential strengths and pitfalls. A potential strength was data sharing and collective improvement in outcomes, more than could be achieved by simple “absorption” of a smaller institution by a larger one. A notable pitfall was mission discordance between the partnering institutions. When the partners implicitly differed in their goals as a partnership, the joint programme tended to fail to achieve either set of goals, leading to joint programme discontinuation.
We explore these ideas further by reporting results of a second survey we conducted of United States programmes ten years after our first survey study. Though the two surveys were not identical in question content, they overlapped sufficiently in content to evaluate trends in joint programme constructs over time.
Materials and methods
Survey design
We designed a survey composed of multiple-choice questions addressed to paediatric cardiac surgeons in the United States of America on the 2019 mail list of the Congenital Heart Surgeon’s Society. Results were collected between September 2019 and January 2020. The first 23 questions were designed to characterise the respondent surgeon’s primary programme or institution of practice. If the respondent indicated participation in a joint programme, they were instructed to answer 42 additional questions for each “partner” hospital with which they held a joint programme. Respondents were to consider the home institution of the older, busier programme as the “primary” institution and all other joint programme hospital participants as “partner” institutions to avoid confusion in answering questions. Respondents were asked if their joint programme had ended in the last 8 years and, if so, were asked an additional six questions about discontinuation of the programme. This discontinuation included cessation of a relationship with one partner hospital while maintaining a joint programme with another (third) partner hospital. The final question asked the respondent to choose, among four choices, their favoured model for delivery of optimal cardiac surgical care in the United States of America.
Questions included in the survey were designed to characterise paediatric cardiac surgery programmes, whether lone, partnered, and those formerly partnered. Fundamental characteristics such as number of beds, case volumes, case complexity, geographic relation to other (partner and/or non-partner) programmes, demographics, number of surgeons, and primary administrative structure were elicited. Additionally, respondents were asked about availability of specific clinical services, academic involvement, clinical administrative structure at partner hospitals, inter-institutional communication, and clinical coverage structure. Finally, methods of outcome reporting, programme objectives, and perceived programme success were queried.
Incomplete surveys, defined as those with greater than five required questions left unanswered, were excluded. Surveys completed by nursing and administrative staff, clinical and research fellows, residents, and clinically inactive attending surgeons were also excluded. If two responses were submitted from two individuals from the same institution, responses were compared to ensure consistency. In all such cases, responses were found to be consistent and one response was used in our analysis. If two responses were submitted from two different individuals from the same joint programme, but two different institutions within that joint programme, the response from the primary institution was used in our analysis.
Statistical analysis
For each question, percentage of respondents selecting each choice was calculated. Differences in the proportional distribution were evaluated using the chi square or (when a given option was selected <5 times) Fisher exact test with p-values less than 0.05 considered statistically significant.
Results
We received 56 responses, of which 47 were from clinically active attending paediatric cardiac surgeons. Of those 47 individuals, 44 answered the survey to completion. Of the 44 completed surveys, 10 were excluded because the surgeon was from the same institution or joint programme as another responding surgeon. As such, we analyzed 34 responses, representing 34 surgeons and 34 institutions (Figure 1).
Of the 34 institutions, 14 (41%) reported having a current or former joint programme in the last 8 years. Of those 14 institutions, 12 have joint programmes currently and 2 were discontinued in the last 8 years. The 14 joint programmes included 8 with one partner hospital and 6 with two partner hospitals, for a total of 34 hospitals participating in a joint programme. About 86% of joint programmes felt as though the relationship between the primary institution and partner institutions was “mother daughter” and 14% of joint programmes felt the relationship to be equal in all respects. Six of the current 12 joint programmes and 1 of the 2 discontinued joint programmes existed or have existed for 10 years or more. The distributions of hospital types housing primary and partner participants are shown in Figure 2a and differ significantly (p < 0.0004).

Figure 1. Response distribution, inclusions, and exclusions.
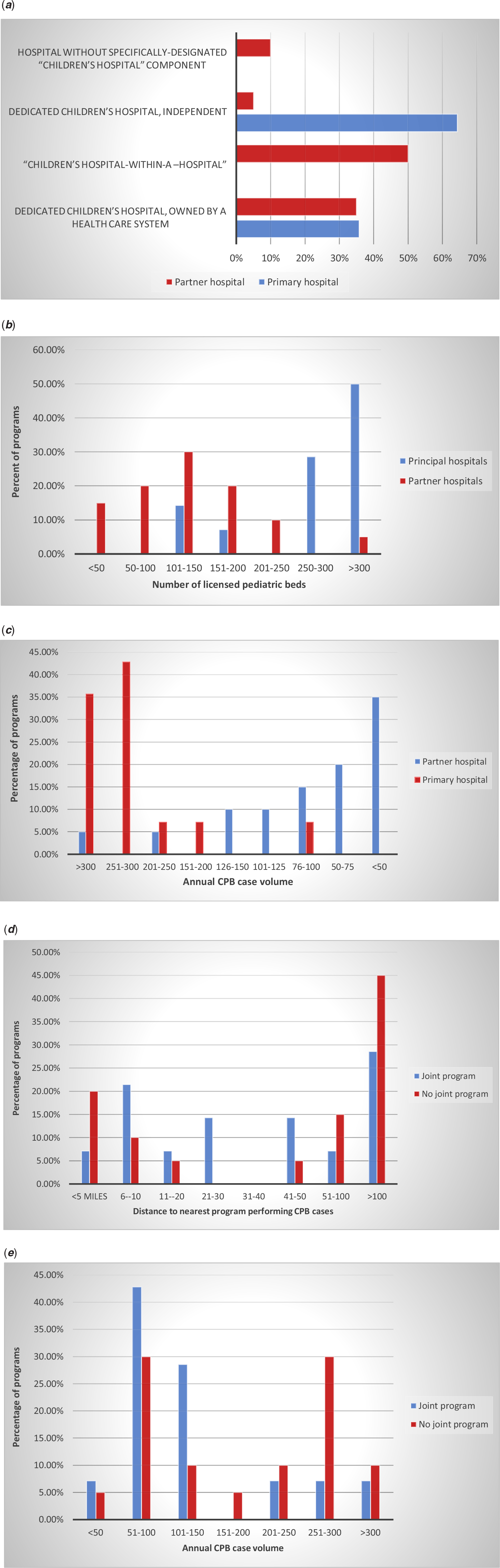
Figure 2. (a) Distribution of type of hospital (as percentage of all joint programmes). (b) Distribution of number of licensed paediatric beds (as percentage of all joint programmes). (c) Distribution of annual volume of cardiopulmonary bypass cases (as percentage of all joint programmes). (d) Distribution of distance to nearest programme performing cardiopulmonary bypass cases. (e) Annual cardiopulmonary case volume of the nearest hospital performing CPB cases.
The numbers of licensed paediatric beds in primary and partner hospitals are shown in Figure 2b and also differ significantly (p < 0.002). 50% of primary hospitals had more than 300 paediatric beds, whereas only 1 partner hospital (5%) had more than 300 paediatric beds; 65% of partner hospitals had 150 or fewer paediatric beds, as opposed to only 14% of primary hospitals.
The distributions of annual cardiopulmonary bypass case volumes for primary and partner hospitals are shown in Figure 2c and differ significantly (p < 0.001) with 35% of partner hospitals completing fewer than 50 CPB cases yearly and only 2 (10%) completing greater than 150 cases yearly; conversely, all but one primary hospital completes more than 150 cases annually. The distributions of annual cardiopulmonary bypass case volumes for hospitals with no history of a joint programme and primary hospitals of joint programmes differ significantly (p = 0.02), with 79% of primary hospitals completing greater than 250 cases annually versus 40% of non-joint programme hospitals (Fig 2e).
Geographic distribution of partner hospitals varied greatly with 20% being within 5 miles and 50% being within 40 miles (Fig 2d). Two partner hospitals were over 500 miles from the primary hospital. Surgical coverage was almost evenly distributed between three structures: all surgeons stationed at primary and travelling to partner when indicated (∼35%), contractually separate groups, one stationed at each hospital (∼29%), and a single contractually united group with separate surgeon teams stationed at each hospital (∼35%). Of the partner hospitals located greater than 40 miles of the primary hospital, 90% had separate surgeon teams stationed at each hospital. Surgeon teams at six of the nine partner hospitals with separate surgical teams were contractually part of the surgeon group of the primary hospital, while three of the teams were a contractually separate group from the surgeon group at the primary hospital.
Cardiology coverage was mostly provided by contractually separate groups stationed at each hospital (85%); cardiac anaesthesia and ICU coverage structure mirrored cardiology coverage structure and no cardiology, anaesthesia, or ICU coverage was provided by individual providers travelling from the primary centre to the partner institution. Perfusion services were mostly provided by separate groups stationed at each hospital (79%), whereas 21% of partner programmes used perfusionists from the primary hospital.
Case review was conducted in a variety of ways, with 50% of programmes reviewing all cases jointly, 36% reviewing cases only at the hospital at which they arose, and 14% reviewing all cases at the primary hospital. Morbidity and mortality conferences were held either as joint conferences in 50% of programmes or as separate conferences in 50% of programmes. Of programmes in which both hospitals report to the STS database, 50% submit one joint report and 50% submit two separate reports.
71% of joint programmes limit the complexity of cases performed at partner hospitals. The highest complexity partner hospitals performed varied: STAT 5 (30%), STAT 4 (10%), STAT 3 (35%), STAT 2 (20%), and STAT 1 (5%). Of the joint programmes that had no surgeon stationed at the partner hospital (total of seven partner hospitals), no partner hospital performed STAT 4 or 5 cases, 3 performed STAT 3 cases, and 4 performed STAT 2 cases. Of the four joint programmes that discontinued their relationship with a partner hospital, three intentionally limited case complexity of the partner hospital; of those three programmes, all the former partner programmes now perform cardiopulmonary bypass cases with no limit on complexity. Respondent surgeons reported that, among the partner hospitals that performed STAT 4–5 cases, 56% had outcomes that were not significantly different than the primary hospital and 44% had outcomes that were not as good as those of the primary hospital. The distribution of post-operative length of stay for the arterial switch procedure was not significantly different between the primary and partner institution in programmes where both the primary and partner hospital performed the procedure.
In total, 80% of primary hospitals have formal contracts with partner hospitals. Approximately 71% of joint programmes have separate programme administrators at each hospital, 14% have one administrator for all partner hospitals, and 14% have no programme administrator and are solely physician managed. Four of the joint programmes (29%) had administrative fees associated with the programme, four (29%) had no administrative fee, and six (42%) respondents did not know if their programme had an administrative fee. Annual dministrative fees ranged from less than $100,000 to $1.0–2.0 million.
Respondents were asked to select one of four objectives most closely resembling that of their joint programme. Approximately 57% selected “to improve one or the other hospital's outcomes,” and 43% selected “ultimately increase the referral base of cases to one hospital.” Of the eight programmes whose main objective was to improve outcomes, six believed the joint programme met that objective and two felt that could never be determined. Of the six programmes whose main objective was to increase referrals to one hospital, five believed the joint programme met that objective and one believed it did not. Among surgeons participating in a joint programme, 71% believe that the joint programme resulted in better outcomes than two separate programmes, 21% disagree, and 8% do not know whether or not that is true. Additionally, 33% of respondents felt that their joint programme resulted in both better outcomes and reduced cost of congenital cardiac care.
While duplicated responses were excluded from most of this analysis, all completed responses were examined to evaluate individual surgeons’ perceptions of the best way to deliver paediatric cardiac surgical care, which was elucidated by the final question of the survey. Twenty-seven of the responses belonged to surgeons whose programmes have never participated in a joint programme and seventeen of the responses belonged to surgeons whose programmes have participated in a joint programme (either currently or previously). Of the 27 surgeons whose programmes have not been part of a joint programme, 48% are in favor of regionalisation, 33% are in favour of joint programmes, and 19% are in favour of allowing market forces to determine the structure to deliver paediatric cardiac surgical care. Of the 17 surgeons whose programmes have participated in a joint programme, 76% are in favour of regionalisation, 18% are in favour of joint programmes, and 6% are in favour of market forces. Of the 10 respondents reporting that their joint programme had better outcomes than two separate programmes, 7 believed that the optimal care delivery model nonetheless would be achieved through regionalisation; 3 believed that optimal care delivery would be achieved through the formation of joint programmes.
Discussion
The results of our follow-up survey indicate that joint programmes have remained relatively common, with a similar proportion of current/former joint programmes as our prior survey. Additionally, the joint programme model has evidence demonstrating longevity and sustainability, with 50% of current joint programmes existing for >10 years, compared to only 26% of those that existed in 2011. Of note, although we identified many joint programmes, the focus of this study was not to determine the actual prevalence of joint programmes, but rather to obtain enough responses to analyse the characteristics of joint programmes. The case volume distributions of primary and partner hospitals were like those in our prior survey, with primary institutions being considered “high- volume” centres and partner hospitals performing far fewer, often fewer than 50 cases per year. The most common relationship between primary and partner institutions appears to have remained “mother–daughter,” which explains or may be the result of case distribution, both in volume and complexity.
Joint programmes are an option not only to improve access to care in more remote areas but also a way to consolidate resources and streamline services in areas with large populations and multiple existing programmes in a small geographic area, potentially reducing costs and improving outcomes. The main alternative to joint programmes is regionalisation of congenital cardiac surgical care. The main driver of this approach is the purported positive association between paediatric surgery case volume and outcomes. Reference Ghandour, Vervoort, Welke and Karamlou14 A statistical analysis by Sakai-Bizmark and coworkers using administrative databases noted outcomes were improved particularly when patients were “virtually” moved from low volume (lowest third) centres to high-volume (highest third) centres; similar improvement was observed in cost of care, but not length of stay. Reference Sakai-Bizmark, Mena and Kumamaru15 Interestingly, the largest proportion of cases that were “virtually” moved to larger centres in this study were low- and medium-risk cases, which is unexpected, as one may predict the greatest outcome/volume advantage would be recognised in the highest risk cases that often require greater expertise and experience. An interesting study simulating regionalisation found that mortality benefits began to be observed when patients at hospitals performing <60 cases were moved to higher volume centres, an effect that plateaued at case volumes >243. Reference Welke, Pasquali and Lin16 Despite these benefits, regionalisation resulted in statistically significant increases in travel distance, which was noted by authors to have potential for disproportionately affecting patients with fewer resources. Additionally, in the study of Sakai-Bizmark and coworkers, the authors suggested that increased length of stay in high-volume programmes was associated with decreased access. As such, joint programmes represent a potential model that possesses the benefits related to resource consolidation of regionalisation while retaining the current access to care, thus avoiding exacerbation of socio-economic disparities in healthcare access.
The success of a joint programme depends on the implementation of and assiduous adherence to a structure and set of processes particular to such a collaboration. Specifying what types and complexity of catheter-based and surgical procedures will be performed at each member programme can be a particularly sensitive issue but must be decided upon based on the common goal of optimising outcomes, access and cost. Other aspects of structure and process, such as joint conferencing, data sharing, tight cross coverage, and resource sharing, were discussed in our prior study. These factors continue to be present among the programmes analysed in the present study, although with some variability. Perhaps concerning is the fact that only half of the joint programmes had a joint case (or “cath”) conference or a joint morbidity and mortality conference. Joint conferencing would seem to be beneficial to the objectives of joint programme and straightforward to implement. A finding not seen in our prior analysis is the submission of a single, “joint” report to the Society of Thoracic Surgeons (STS) Congenital Database by several of the joint programmes. Permission by the STS to do so was granted in the interim between our two studies, implicitly illustrating the STS’s support of the joint programme model. Most of the surgeons who were participating in a joint programme with that goal felt that the joint programme achieved the stated goals. Commitment to the joint programme with resource allocation, unified goals, and shared accountability is critical to their success; current joint programmes appear to be taking the steps necessary to foster collaborative success.
Our survey also suggests that a possible outcome of some joint programmes is the development or maturation of struggling or initially less robust congenital cardiac surgery programmes. As highlighted above, within the four defunct joint programmes, three of the partner hospitals had complexity limitations while participating in the joint programme, but now operate without limits on case complexity. While continued collaboration likely has its benefits, it is not unreasonable to imagine that collaboration, mentorship, development of mature, evidence-based protocols, and quality improvement initiatives directed by a more experienced institution could give rise to a safe, effective, independently functioning congenital cardiac programme; however, this claim should be taken with caution, as the effect of sudden reduction in resources previously provided by a partner institution may have significant long-term effects on the newly independent programme that are not immediately apparent.
Interestingly, despite the opinion by most respondents that their joint programme achieved its stated goals and improved outcomes, the perceived ideal model for delivery of congenital cardiac care among patients in joint programmes was regionalisation. The logistics and coordination involved in creating a unified programme of care from two previously independent institutions may be cumbersome. Just as it is important to have appropriate non-surgeon providers and services (anaesthesia, cardiology, critical care, and perfusion) to successfully operate a single programme, it is equally important to have the administrative and quality support to coordinate unified protocols and standards of care, between institutions of a joint programme. A solution that may address the frustrations associated with coordinating a multi-institution joint programme is shifting of administrative and oversight personnel at each individual institution to roles on a unified committee overseeing the joint programme. In this way, accountability and communication are improved, standards and protocols are clearly established, and formal structure representing the interests of the joint programme evolves.
Though every effort was made to clearly characterise as many aspects as possible of joint programmes and their evolution over the last decade, our study is limited by factors such as sample size and bias. Our survey was sent to all practicing paediatric cardiothoracic surgeons listed in the CHSS database, but only 34 qualifying institutions were included, representing approximately 25–30% of existing programmes in the United States of America. This result was likely related to the large number of questions in the survey. Our decision was to balance the response rate with the depth and breadth of information we felt we needed to adequately characterise joint programmes. Importantly, we did not attempt to demonstrate that the joint programme model improves outcomes, cost, or access, as this was not part of our study design. We chose only to present descriptive data on the current characteristics of joint programme in paediatric cardiac surgery in the United States of America and suggest that, if properly structured, a joint programme may have these benefits.
Acknowledgements
The authors would like to thank all of the respondents to the survey, as this study would not be possible without their participation.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
The authors report no conflicts of interest.
Disclosures
No disclosures.





