Recurrent laryngeal nerve injury leading to vocal cord paralysis is a complication occurring with trauma during neck or chest surgery, viral infection, tumour compression, a neurologic condition or indirectly from intubation. The left recurrent laryngeal nerve is more commonly injured as it lies closer to the surgical plane of dissection, especially during aortic arch procedures (Fig 1). Reference Lee1, Reference Truong, Messner and Kerschner2 Injury is suspected when symptoms such as hoarseness, difficulty speaking and swallowing occur, which can result in aspiration necessitating nasogastric or gastric tube feedings, increasing morbidity and hospital length of stay. Reference Dewan, Cephus, Owczarzak and Ocampo3 The reported incidence after congenital cardiac surgery varies widely, with one study reporting an incidence of 1.7–67%. Reference Alfares, Hayes and Ansari4 Another single-centre study found an incidence of only 1.05%. Reference Kobayashi, Turner and Humes5 Each of these studies focused on different procedures and patient populations. Reported recovery rates following recurrent laryngeal nerve injury also encompass a broad range from 0 to 82%. Reference Truong, Messner and Kerschner2 In a multicentre retrospective study of multiple cardiac surgeries, the lowest recovery rates were noted after surgical patent ductus arteriosus ligation. Reference Truong, Messner and Kerschner2
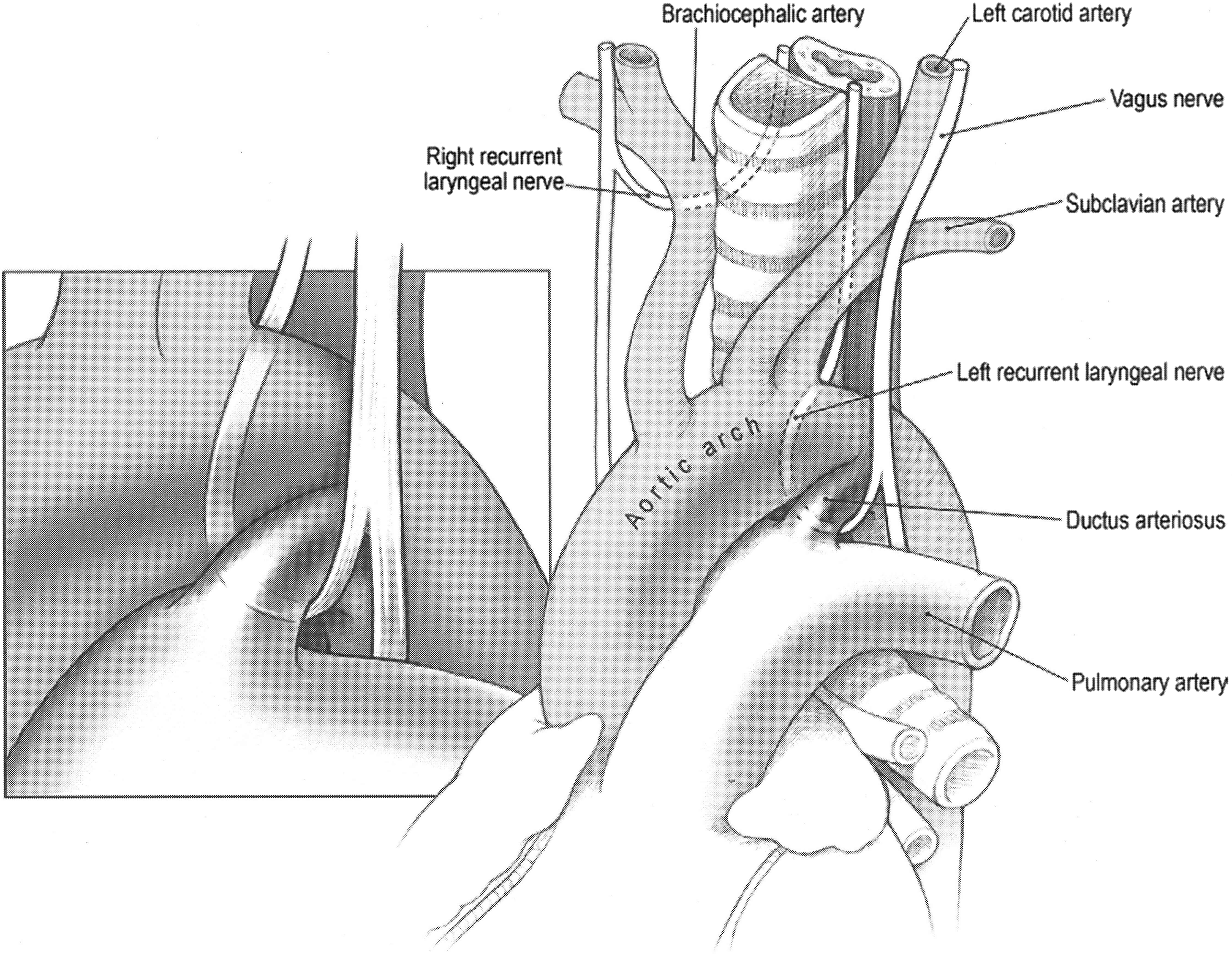
Figure 1. Anatomical relationship of the left recurrent laryngeal nerve to the aortic arch, pulmonary artery, and ductus arteriosus. (Image reprinted with permission from McGraw Hill. All rights reserved).
Single-centre case reports describing recurrent laryngeal nerve injury following interventional congenital cardiac catheterisations have been published. Reference Kobayashi, Turner and Humes5–Reference Baek, Chung, Kim, Song, Bae and Noh7 Two single-centre retrospective analyses of patent ductus arteriosus occlusion procedures reported recurrent laryngeal nerve injury as a rare complication. Reference Javois, Patel, Roberson and Husayni8,Reference Liang, Ko, Huang, Huang and Niu9 The left recurrent laryngeal nerve is located between the left pulmonary artery and patent ductus arteriosus and is vulnerable to injury during procedures on these structures. To date, no multicentre studies have been performed to determine the incidence of proven left recurrent laryngeal nerve injury after interventional congenital cardiac catheterisation procedures involving left pulmonary artery stenting or patent ductus arteriosus occlusion.
Materials and methods
The Congenital Cardiovascular Interventional Study Consortium is a group of interventional paediatric cardiologists involved in the scientific study of interventional cardiovascular care for the CHD population. Members were queried regarding their experience of confirmed vocal cord paralysis secondary to presumed left recurrent laryngeal nerve injury following interventional congenital catheterisation procedures. Twelve centres responded and performed their own retrospective database analysis ranging from 6 to 26 years in length. They submitted de-identified data on patients who had documented vocal cord paralysis confirmed via direct laryngoscopy after left pulmonary artery endovascular stenting, patent ductus arteriosus device implantation or a combination of both procedures performed either concurrently or at different times. The total number of these procedures performed at each institution was documented for statistical comparison. In addition, time to symptom resolution was also reported. The institutional review board at each participating institution approved the study and waived informed consent due to the retrospective design.
Odds ratios for recurrent laryngeal nerve injury were calculated comparing each interventional procedure (left pulmonary artery stenting, patent ductus arteriosus device closure and the combination) and cardiothoracic surgery. The incidence of recurrent laryngeal nerve following cardiothoracic surgery used for comparison was 1.05% determined by Alfares et al Reference Alfares, Hayes and Ansari4 (n of 3036, with 32 patients confirmed to have recurrent laryngeal nerve injury). Mantel–Haenszel odds ratios were calculated to compare the interventional procedures to each other to account for institutions as a confounding variable. A p-value < 0.05 was considered statistically significant. Statistics were calculated using SAS® 9.4 software (SAS Institute, Cary, NC, USA).
Results
A total of 5364 patients from 12 institutions underwent patent ductus arteriosus device closure, left pulmonary artery stenting or a combination of the two procedures (Table 1). A total of 4001 patients underwent patent ductus arteriosus device closure. Of them, two patients were confirmed to have left vocal cord paralysis, representing a 0.05% incidence for that population. A total of 1337 patients underwent left pulmonary artery stenting and six patients (0.45%) had confirmed vocal cord paralysis. The group of patients who had both procedures performed had the highest incidence of vocal cord paralysis with it occurring in 4 of 26 patients (15.4%). Of the two patients with vocal cord paralysis after patent ductus arteriosus device closure, one had resolution of symptoms in 4 months and the other resolution in 6.5 months. Five of the six left pulmonary artery stent patients had resolution of vocal cord paralysis within 2 years of the procedure, but one remained symptomatic 21 months after the procedure. This patient was diagnosed with isolated congenital left pulmonary artery stenosis at 4 years old with no previous history of cardiac interventions. Reference Kobayashi, Turner and Humes5 He was previously described in a case report. Reference Kobayashi, Turner and Humes5 The four patients who underwent both patent ductus arteriosus device closure and left pulmonary artery stenting had resolution within a year, with one requiring laryngoplasty and another requiring left vocal cord injection. Overall, the rate of left vocal cord paralysis recovery in our study population was 92%. Table 2 describes characteristics of the affected patients.
Table 1. Data by institution

LPA = left pulmonary artery; PDA = patent ductus arteriosus
The boldface values are to more easily identify affected cases as there were so few
Table 2. Characteristics of affected patients

LPA = left pulmonary artery; PDA = patent ductus arteriosus
Statistical results
In Table 3, procedure #1 was considered the exposure group during calculation of the odds ratios. The MH odds ratios indicate that the combination of patent ductus arteriosus device closure and left pulmonary artery stent placement carried a higher risk of recurrent laryngeal nerve injury versus patent ductus arteriosus closure and left pulmonary artery stenting alone. Left pulmonary artery stenting carried a higher risk of recurrent laryngeal nerve injury versus patent ductus arteriosus device closure. When compared to surgery, patent ductus arteriosus device closure and left pulmonary artery stent placement individually had a lower risk of recurrent laryngeal nerve injury, although the odds ratio for left pulmonary artery stent versus surgery fell just outside statistical significance. The combination of patent ductus arteriosus device closure and left pulmonary artery stent placement, however, carried a higher risk of recurrent laryngeal nerve injury versus surgery. While the 95% confidence interval for all the odds ratios is very wide, the p-value indicates statistical significance for all save left pulmonary artery versus surgery.
Table 3. Statistical results

LPA = left pulmonary artery; PDA = patent ductus arteriosus
Discussion
Vocal cord paralysis secondary to recurrent laryngeal nerve injury is a well-known complication following cardiothoracic surgery, and it is included on The Society of Thoracic Surgeons Data Collection Form in the complication section as “vocal cord dysfunction (possible recurrent laryngeal nerve injury).” 10 However, it is not included in the reports received by participating institutions. 10 It has a significantly variable reported incidence, ranging from 1.05 to 67%. Reference Dewan, Cephus, Owczarzak and Ocampo3,Reference Alfares, Hayes and Ansari4 When analysing this variation, it is important to keep in mind that these studies differ significantly with respect to procedures included and patient populations studied. For example, the study reporting a 67% incidence of recurrent laryngeal nerve injury was limited to extremely low birth weight infants undergoing surgical patent ductus arteriosus closure. Reference Malcolm, Hornik, Evans, Smith and Cotten11 One single-centre study found that of the neonatal patients who developed vocal cord paralysis after congenital heart surgery, 45% required surgical gastrostomy tube placement, which significantly prolonged hospital stay. Reference Alfares, Hayes and Ansari4 In addition, 13% were sent home with nasogastric tube feedings. Reference Alfares, Hayes and Ansari4 Airway operations, such as tracheostomy or airway reconstruction, were performed in 23% of the patients. Reference Alfares, Hayes and Ansari4 Recurrent laryngeal nerve injury is also a possible complication of interventional catheterisation procedures, specifically patent ductus arteriosus device closure and left pulmonary artery stenting due to their anatomic proximity to the left recurrent laryngeal nerve.
Kobayashi et al Reference Kobayashi, Turner and Humes5 reported a 10-year-old girl who developed persistent left vocal cord paralysis thought to be due to left recurrent laryngeal nerve injury after left pulmonary artery stent placement. Assaqqat et al Reference Assaqqat, Siblini and Al Fadley6 reported left recurrent laryngeal nerve injury after simultaneous left pulmonary artery stent placement and patent ductus arteriosus coil occlusion. A 10-year-old boy developed hoarseness after left pulmonary artery stent placement and was found to have left recurrent laryngeal nerve injury and left vocal cord paralysis; of note, the patient had a patent ductus arteriosus device closure at the age of 1 year. Reference Baek, Chung, Kim, Song, Bae and Noh7 On literature review, only two retrospective studies were found. Javois et al Reference Javois, Patel, Roberson and Husayni8 performed a single-centre retrospective review of all patients who underwent patent ductus arteriosus device occlusion over 7 years. Out of 196 patients that underwent successful patent ductus arteriosus device occlusion, 1 (0.5%) had cough and hoarseness. Left vocal cord paralysis was discovered upon fibreoptic laryngoscopy and repeat laryngoscopy 6 months post-procedure continued to demonstrate left vocal cord paralysis.
Liang et al Reference Liang, Ko, Huang, Huang and Niu9 performed a single-centre retrospective chart review of patients who underwent Gianturco coil embolisation of the patent ductus arteriosus over a period of 3 years. Of the 75 patients identified, 3 (4%) had hoarseness and were confirmed to have left vocal cord paralysis by fibreoptic bronchoscopy. Of note, all three patients were less than 1 year old. One year post-procedure, two of the three patients no longer had hoarseness.
Our multicentre retrospective chart review indicates that the incidence of left recurrent laryngeal nerve injury during interventional congenital catheterisation procedures is very low, especially when compared to the reported incidence following surgical procedures. This finding is consistent with the data found during our literature review. However, the exception is the combination of left pulmonary artery stenting and patent ductus arteriosus device closure, which had a significantly higher incidence, even compared to surgical procedures. The aetiology of this phenomenon may be due to the location of the left recurrent laryngeal nerve and its proximity to both the left pulmonary artery and the patent ductus arteriosus. The anatomy may lend itself to the left pulmonary artery stent and patent ductus arteriosus device forming a vice around the nerve. Additional studies will be required to determine the effects of different factors on the incidence of recurrent laryngeal nerve injury. Some devices are more rigid than others and may exert more force on the nerve. Size of the device, location of the left pulmonary artery stenosis and patent ductus arteriosus classification are just a few of the factors that should be analysed. For patients that underwent the combination of both procedures, perhaps the sequence of procedures may also play a role.
While multiple affected patients were identified in our study, there were not enough to perform any statistical analysis to provide further insight into what factors may contribute to recurrent laryngeal nerve injury. However, we did note that two of the injuries occurred after dilation of the left pulmonary artery stent, suggesting that size of the device may have a mechanical effect on the recurrent laryngeal nerve. The ages of the patient and the devices utilised varied widely, indicating further investigation is necessary to elucidate their role in the risk of recurrent laryngeal nerve injury.
Of note, our study indicated a better recovery rate than surgery, 92 versus 61%, respectively. Reference Alfares, Hayes and Ansari4 Another study demonstrated a 70% recovery rate in neonates with vocal cord dysfunction after aortic arch repair. Reference Pourmoghadam, DeCampli and Ruzmetov12 Literature review demonstrated only 1 case report of a patient who developed vocal cord paralysis secondary to recurrent laryngeal nerve injury after re-stenting of coarctation of the aorta and the patient recovered with no intervention within 6 months. Reference Fürniss, Hummel, Stiller and Grohmann13 While the statistical significance of this finding was not calculated, it raises the possibility that recurrent laryngeal nerve injury secondary to interventional congenital catheterisation procedures is of lower risk than cardiothoracic surgery. Interventional procedures may cause a stretch or compression phenomenon of the left recurrent laryngeal nerve versus true injury during surgical procedures. Another question to explore is whether patients with left recurrent laryngeal nerve injury following cardiothoracic surgery require more interventions for resolution compared to patients who underwent catheter based interventions.
Limitations of this study include a variation in time span of chart reviews across institutions. As only vocal cord paralysis cases confirmed by direct laryngoscopy were included, asymptomatic cases were likely excluded, meaning the true incidence may have been underestimated. A recent meta-analysis demonstrated that the incidence of vocal cord paralysis was significantly higher when all post-operative patients underwent laryngoscopy instead of only symptomatic patients. Reference Dewan, Cephus, Owczarzak and Ocampo3 However, the clinical significance of asymptomatic patients with vocal cord paralysis is low as they do not require additional procedures or a potential increased length of stay. While all of the 95% confidence intervals were broad demonstrating a lack of precision in the results, each one save left pulmonary artery stenting versus surgery interventions did not overlap the null value of one. Further study will be required to illuminate what is likely a multifactorial aetiology of recurrent laryngeal nerve injury risk with regard to interventional congenital catheterisation procedures.
Conclusion
Recurrent laryngeal nerve injury is a possible, albeit rare, consequence of left pulmonary artery stent placement and patent ductus arteriosus device closure. However, particular attention should be paid to those situations when both the left pulmonary artery and patent ductus arteriosus require intervention as the incidence was highest in these patients. Discussion of the potential risk of recurrent laryngeal nerve injury and vocal cord paralysis should be incorporated into the informed consent process when any of these procedures are being performed either individually or simultaneously. Additional research should focus on identifying contributing factors so that they, and therefore the risk of recurrent laryngeal nerve injury, can be avoided.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
Dr Shahrier has no relationships to disclose. Dr Chinchilli has no relationships to disclose. Dr Qureshi is a consultant for WL Gore and Associates, Medtronic, Edwards LifeSciences and Abiomed Inc. Dr Prieto has no relationships to disclose. Dr Levi is a consultant for Edwards and Medtronic. Dr Boe has no relationships to disclose. Dr Turner has no relationships to disclose. Dr Rubio has no relationships to disclose. Dr Trucco has no relationships to disclose. Dr Devanagondi has no relationships to disclose. Dr Law has no relationships to disclose. Dr Bass has no relationships to disclose. Dr Pihkala has no relationships to disclose. Dr Weber is a proctor for structural heart device implantation at Abbot-St. Jude Medical.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees of Penn State College of Medicine, Baylor College of Medicine, Nicklaus Children’s Hospital, University of California Los Angeles, Nationwide Children’s Hospital, Children’s Hospital of Michigan, Seattle Children’s Hospital, UPMC Children’s Hospital of Pittsubrgh, Golisano Children’s Hospital, University of Alabama at Birmingham, Children’s Minnesota and University of Helsinki.







