Infants with CHD who require surgical intervention often experience difficulties with oral feeding. Reference Hill, Tey and Jung1 In particular, infants with single-ventricle physiology have been found to be at risk for poor feeding. Reference Indramohan, Pedigo, Rostoker, Cambare, Grogan and Federman2 In a recent study of single-ventricle patients, 57% required tube feeding at the time of discharge from the neonatal hospitalisation and 39% needed a feeding tube at one year of age. Reference Sagiv, Tjoeng and Davis3 In another chart review study of neonates requiring cardiac surgery, oral aversion was present in 23.6% at one year of life, and an additional 18.5% experienced other feeding difficulties at one year. Reference Goldstein, Watkins, Lowery and Yu4 Difficulties with feeding impact the quality of life of the infant and their families, parent–child bonding, nutrition, growth, and neurodevelopment. Reference Jones, Desai and Fogel5,Reference Steward, Ryan-Wenger, Harrison and Pridham6 Historically, a lack of valid and reliable feeding assessment tools has limited research related to feeding in this population. Reference Pados7 Recent development of feeding assessment tools with adequate psychometric properties has allowed for better objective assessment of problem feeding. Reference Pados7 Additionally, much of the research on feeding in infants with CHD has focused on early infancy, with little research on feeding in later infancy when solid foods are introduced. The purpose of this study was to describe the feeding skills and behaviours of infants with CHD at 6–12 months of age and explore the relationships between feeding skills, behaviours, symptoms of gastrointestinal distress and gastroesophageal reflux.
Materials and method
This was a descriptive, longitudinal study. Eligible infants were identified during their neonatal hospitalisation at a children’s hospital in the midwestern United States between May 2019 and January 2020. Eligible infants were diagnosed with a CHD requiring surgical intervention within the first month of life and were born full-term (≥37 weeks gestation). All infants were fed according to a standard feeding protocol in terms of amounts and advancement in quantities of milk and adjusted based on infant tolerance. Adult parents (at least 18 years old) of eligible infants were invited to participate in a series of online surveys when their infant was 2, 4, 6, 8, 10, and 12 months of age. In addition, demographic information and information about the infant’s diagnoses, number of functional ventricles, and surgical intervention(s) were collected. In this analysis, we present data from the 6-, 8-, 10-, and 12-month surveys, focused on infants eating solid foods. At each survey time point, parents completed four measures.
Data were collected and managed using the Research Electronic Data Capture platform hosted at Boston College. Reference Harris, Taylor and Thielke8,Reference Harris, Taylor and Minor9 The Research Electronic Data Capture platform is a secure, web-based software platform designed to support data capture for research studies, providing 1) an intuitive interface for validated data capture; 2) audit trails for tracking data manipulation and export procedures; 3) automatic export procedures for seamless data downloads to common statistical packages; and 4) procedures for data integration and interoperability with external sources.
Measures
Pediatric Eating Assessment Tool. Reference Pados, Thoyre and Park10-Reference Thoyre, Pados, Park, Estrem, McComish and Hodges12 The Pediatric Eating Assessment Tool is a 78-item, parent-reported measure of symptoms of problematic feeding related to solid-food eating in infants and children 6 months to 7 years old. Reference Thoyre, Pados, Park, Estrem, McComish and Hodges12 Specifically, this tool measures a child’s feeding behaviours and their willingness to eat certain foods. Items are rated on a six-point Likert scale, with possible range of scores between 0 and 390. Higher scores indicate more symptoms of problematic feeding. Scores above the 90th percentile based on the reference values were defined as problematic feeding behaviours. Reference Harris, Taylor and Thielke8
There are four subscales on the Pediatric Eating Assessment Tool: Physiologic Symptoms, Problematic Mealtime Behaviors, Selective/Restrictive Eating, and Oral Processing. The Physiologic Symptoms subscale includes questions about behaviours such as coughing during or after eating, breathing faster or harder when eating, and becoming pale or bluish around the lips during meals. The Problematic Mealtime Behaviors subscale includes questions about behaviours such as avoiding eating, refusing to eat, and showing more stress during meals. The Selective/Restrictive Eating subscale includes questions about behaviours such as willingness to eat foods of different textures and temperatures and willingness to feed themselves. Finally, the Oral Processing subscale includes questions related to behaviours such as storing food in their cheek, preferring smooth foods, and using their fingers to move food in their mouth. The Pediatric Eating Assessment Tool has evidence of acceptable content validity, construct validity, internal consistency reliability, test-retest reliability, and known-groups validity. Reference Thoyre, Pados and Park11,Reference Thoyre, Pados, Park, Estrem, McComish and Hodges12 Reference values have been published based on a sample of full-term infants and children with no known medical diagnoses. Reference Harris, Taylor and Thielke8
Child Oral and Motor Proficiency Scale. Reference Pados, Thoyre and Park13-Reference Park, Pados, Thoyre, Estrem and McComish15 The Child Oral and Motor Proficiency Scale is a 63-item parent-report measure of eating, drinking, and related feeding skills in infants and children 6 months to 7 years old who are being offered solid foods. Reference Park, Pados, Thoyre, Estrem and McComish15 Parents are asked to indicate the child’s ability to perform each skill on a three-point Likert scale, with options of Yes (i.e., established skill; score = 2), Sometimes (i.e., emerging skill; score = 1), and Not Yet (i.e., not yet emerging skill; score = 0). Possible scores range from 0 to 126, with higher scores indicating higher skill level. Child Oral and Motor Proficiency Scale scores below the 10th percentile based on the reference values were defined as problematic feeding skills. Reference Pados, Thoyre and Park13
The Child Oral and Motor Proficiency Scale has four subscales: Basic Movement Patterns, Fundamental Oral Motor Skills, Complex Movement Patterns, and Oral-Motor Coordination. The Basic Movement Patterns subscale includes foundational motor skills, such as holding head up when lying on tummy and bringing a piece of food to the mouth. Fundamental Oral Motor Skills includes questions such as the child’s ability to move their tongue in their mouth from side to side, stick their tongue out, and open their mouth wide enough to accept a spoon. The Complex Movement Patterns subscale includes questions about more complex movements, such as being able to use a filled spoon or fork to bring food to mouth and drink from a straw. Of note, infants 6–9 months corrected gestational age are not expected to achieve any of the skills in the “Complex Movement Patterns” subscale, so this subscale was not applicable to infants at the 6- and 8-month time points. Finally, the Oral-Motor Coordination subscale includes questions about skills such as being able to use the tongue to move food around in the mouth, drink thin liquids without gagging, coughing, or choking, and take a bite of firm food, such as a cracker or cookie. Higher scores on each of the Child Oral and Motor Proficiency subscales indicate more developed skills. The Child Oral and Motor Proficiency Scale has evidence of content validity, construct validity, internal consistency reliability, test-retest reliability, and known-groups validity. Reference Pados, Thoyre, Park, Estrem and McComish14,Reference Park, Pados, Thoyre, Estrem and McComish15 Reference values have been published based on a sample of healthy, full-term born infants and children. Reference Pados, Thoyre and Park13
Infant Gastrointestinal Symptoms Questionnaire. Reference Riley, Trabulsi, Yao, Bevans and DeRusso16 The Infant Gastrointestinal Symptoms Questionnaire is a 13-item measure of gastrointestinal symptoms intended to be completed by parents of infants up to 1 year old. Reference Riley, Trabulsi, Yao, Bevans and DeRusso16 Possible scores on the questionnaire range from 13 to 65. Higher scores indicate more symptoms of gastrointestinal distress (e.g., difficulty passing stool, hard stool, discomfort with spitting up, fussiness, passing excessive gas). The questionnaire has evidence of acceptable internal consistency reliability Reference Riley, Trabulsi, Yao, Bevans and DeRusso16,Reference Pados and Basler17 and concurrent validity. Reference Pados and Basler17
Infant Gastroesophageal Reflux Questionnaire-Revised. Reference Orenstein18,Reference Kleinman, Rothman and Strauss19 The Infant Gastroesophageal Reflux Questionnaire-Revised is a 12-item measure of symptoms of gastroesophageal reflux in infants up to 1 year old. Reference Orenstein18,Reference Kleinman, Rothman and Strauss19 Higher scores indicate more symptoms of gastroesophageal reflux. Possible scores range from 0 to 42. The questionnaire has evidence of acceptable internal consistency reliability, Reference Kleinman, Rothman and Strauss19.Reference Pados and Yamasaki20 test-retest reliability, Reference Kleinman, Rothman and Strauss19 known-groups validity Reference Kleinman, Rothman and Strauss19 and concurrent validity. Reference Pados and Yamasaki20 Permission to use the Infant Gastroesophageal Reflux Questionnaire-Revised was obtained through an agreement with the University of Pittsburgh.
Statistical analysis
We calculated descriptive statistics for the Pediatric Eating Assessment Tool and Child Oral and Motor Proficiency Scale at 6, 8, 10, and 12 months. For the Pediatric Eating Assessment Tool and Child Oral and Motor Proficiency Scale, subscale and total scores were compared to reference values based on the child’s age. Prevalence of problematic feeding behaviours and skills was calculated as the percent of infants at each time point whose Pediatric Eating Assessment Tool or Child Oral and Motor Proficiency Scale scores met the definition of problematic as compared to the reference values. For each of the Child Oral and Motor Proficiency Scale and Pediatric Eating Assessment Tool scores, an overall problem prevalence was calculated as the number of infants who had any subscale or total score that met the definition for a problem out of the total sample at each time point.
To calculate overall incidence of any problem related to feeding, we calculated the percentage of the sample at each time point who either were not feeding by mouth or who met criteria for problematic feeding skills or feeding behaviours. We used an independent samples t-test at each time point to explore differences in Pediatric Eating Assessment Tool and Child Oral and Motor Proficiency Scale subscale and total scores between infants with single and double ventricle physiology.
To explore the relationship between feeding skills, feeding behaviours, gastrointestinal, and gastroesophageal reflux symptoms, we first evaluated the Pediatric Eating Assessment Tool, Child Oral and Motor Proficiency Scale, Infant Gastrointestinal Symptoms Questionnaire, and Infant Gastroesophageal Reflux Questionnaire-Revised scores for normality using the Shapiro-Wilk test. Since the data were generally not normally distributed, we then used the Spearman rank correlation method to explore relationships between these variables. Given the number of comparisons, a Bonferroni adjustment was made and statistical significance was defined as alpha < 0.01.
Results
Sample
Parents of 33 infants with CHD participated in the study. Three of these infants were excluded from this analysis because they were not feeding orally at any of the times the surveys were completed, and therefore, questions about oral feeding were not appropriate. Some parents were not able to provide data at all time points or the data could not be included in this analysis because the infant was not feeding by mouth. Of the 30 infants who were orally feeding at one or more time point, 15 had complete data at all four time points, 4 had data at three of the time points, 6 had data at 3 time points, and 5 had data at one time point. Data from two infants were excluded at certain time points because they were not feeding orally at the time of the survey, but oral feeding data were available at other time points.
All of the infants included in these analyses were receiving some oral feedings by mouth. Of these infants, two were diagnosed with dysphagia and three were diagnosed with aspiration at some point in their first year of life. One infant had a gastrostomy tube that was in place for the 6-, 8-, 10-, and 12-month surveys. The parent of one other infant with a gastrostomy tube only completed the 8-month survey. At 6 months, one infant had a nasogastric tube in place. At 8 months, two had nasogastric tubes, and at 12 months, one had a nasogastric tube.
Within the sample of 30 infants with oral feeding data, 18 (60%) had two functional ventricles, 17 (57%) were male, and 29 (97%) of the respondents identified as the mother of the infant. The parents identified their infant’s race as: White (n = 25 [83%]), Black (n = 1 [4%]), and more than one race (n = 4 [13%]). The majority identified as not Hispanic or Latino (n = 26 [87%]). The infants in this sample had a variety of cardiac lesions, including transposition of the great arteries (n = 8), coarctation of the aorta ± septal defect (n = 4), multiple complex cardiac defects (n = 4), hypoplastic left heart syndrome (n = 4), tetralogy of fallot (n = 4), complete atrioventricular canal (n = 2), hypoplastic aortic arch (n = 1), pulmonary stenosis (n = 1), Shone’s complex (n = 1), and Ebstein anomaly (n = 1). All but one infant required a cardiac intervention prior to discharge from their neonatal hospitalisation. Infants were discharged at a mean of 32.9 days (range 8–177 days). In addition to their cardiac diagnoses, three infants were diagnosed with Trisomy 21, one infant with DiGeorge syndrome, and one infant with Dubin-Johnson syndrome. At 12 months, 62% of the sample was still receiving some of their mother’s milk.
Internal consistency reliability within this sample was acceptable for all of the assessment tools: Pediatric Eating Assessment Tool (Cronbach’s alpha = 0.93), Child Oral and Motor Proficiency Scale (Cronbach’s alpha = 0.96), Infant Gastrointestinal Symptoms Questionnaire (Cronbach’s alpha = 0.72), and Infant Gastroesophageal Reflux Questionnaire-Revised (Cronbach’s alpha = 0.80).
Feeding skills at 6–12 months
The Child Oral and Motor Proficiency Scale total scores increased as eating, drinking, and related skills developed from 6 to 12 months (Table 1). Fundamental Oral Motor Skills subscale scores remained relatively stable at near the highest score possible, while all other subscale scores improved over time (Fig 1). The percentage of the sample that met criteria for problematic feeding skills (i.e., score below the 10th percentile compared to a norm-reference sample at each time point for each subscale and total score) are provided in Table 1. Overall, 95% of the infants in the sample met criteria for problematic feeding skills at 6 months, 32% at 8 months, 67% at 10 months, and 30% at 12 months.
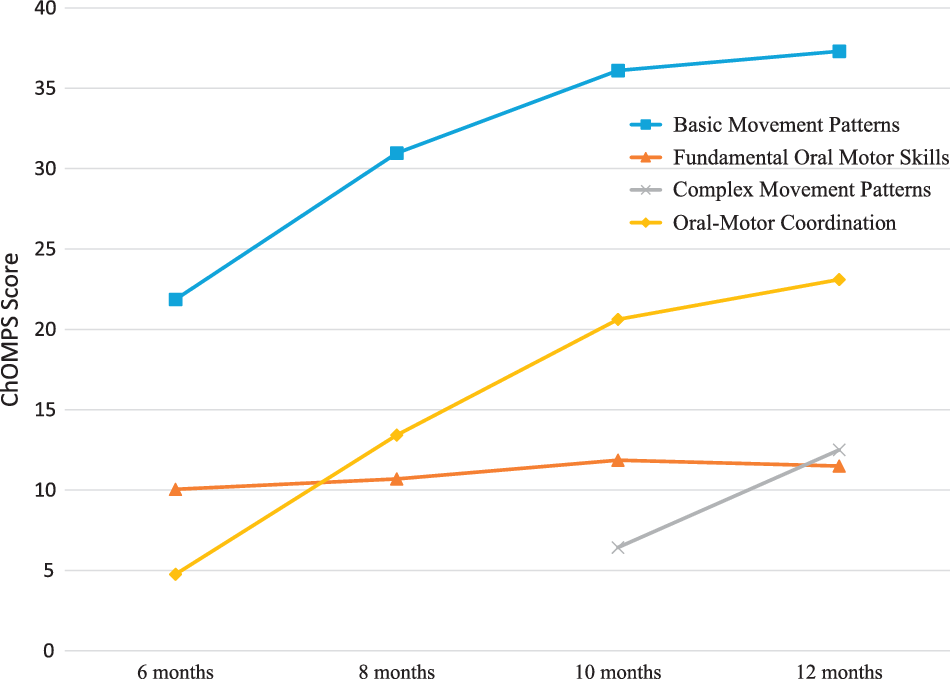
Figure 1. Mean Child Oral and Motor Proficiency (Child Oral and Motor Proficiency Scale) subscale scores over time from 6 to 12 months (n = 30). Higher scores indicate more skill development (i.e., better).
Table 1. ChOMPS and PediEAT scores at 6, 8, 10, and 12 months
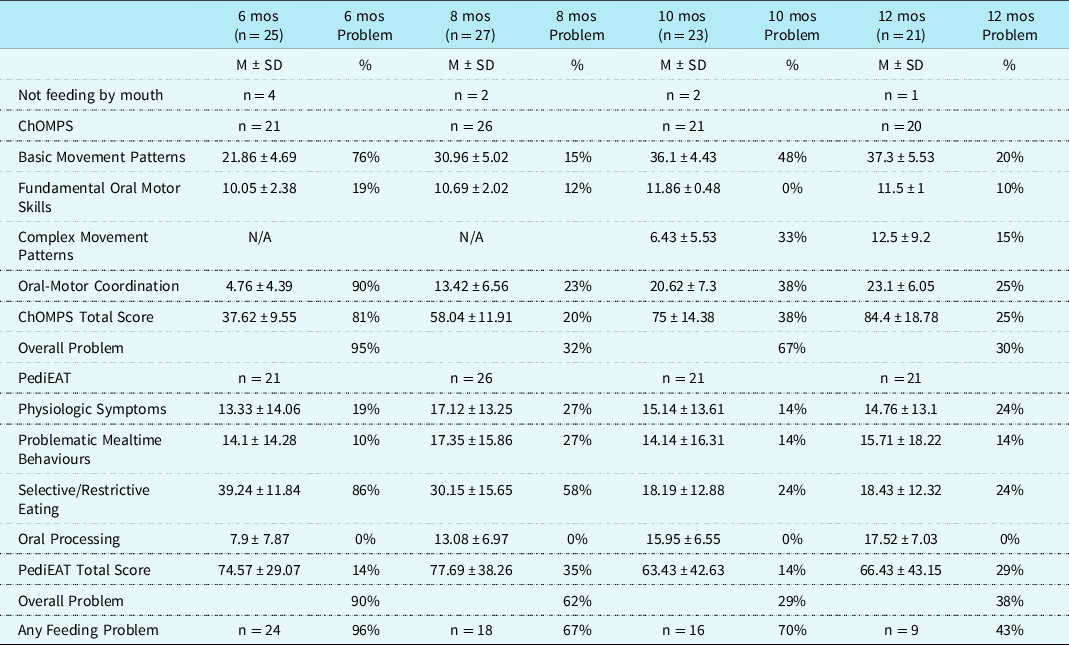
Note. ChOMPS = Child Oral and Motor Proficiency Scale, range of scores 0–126, higher scores indicate more skill (better); mos = months; PediEAT = Pediatric Eating Assessment Tool, range of scores 0–390, higher scores indicate more symptoms of problematic feeding (i.e., worse). The % Problem is defined as the percentage of the sample at each time point that met criteria for problematic feeding based on a norm-reference sample. For the ChOMPS, % Problem is the proportion with eating, drinking, and related skills below the 10th percentile. For the PediEAT, % Problem is the proportion with symptoms of problematic feeding behaviours above the 90th percentile. Any Feeding Problem is defined as the child either not feeding by mouth at that time or meeting criteria for problematic feeding skills or feeding behaviours.
Feeding behaviours at 6–12 months
Pediatric Eating Assessment Tool Total Scores worsened slightly from 6 to 8 months, then improved at 10 months and worsened again slightly at 12 months (Table 1). Selective/Restrictive Eating subscale scores improved from 6 to 10 months, but then stabilised between 10 and 12 months (Fig 2). The Oral Processing subscale worsened over time from 6 to 12 months, while the other subscales of the Pediatric Eating Assessment Tool remained relatively stable. The percentage of the sample that met criteria for problematic feeding behaviours (i.e., score above the 90th percentile compared to a norm-reference sample at each time point for each subscale and total score) are provided in Table 1. Overall, 90% of infants met criteria for a problematic feeding behaviour score at 6 months, 62% at 8 months, 29% at 10 months, and 38% at 12 months.
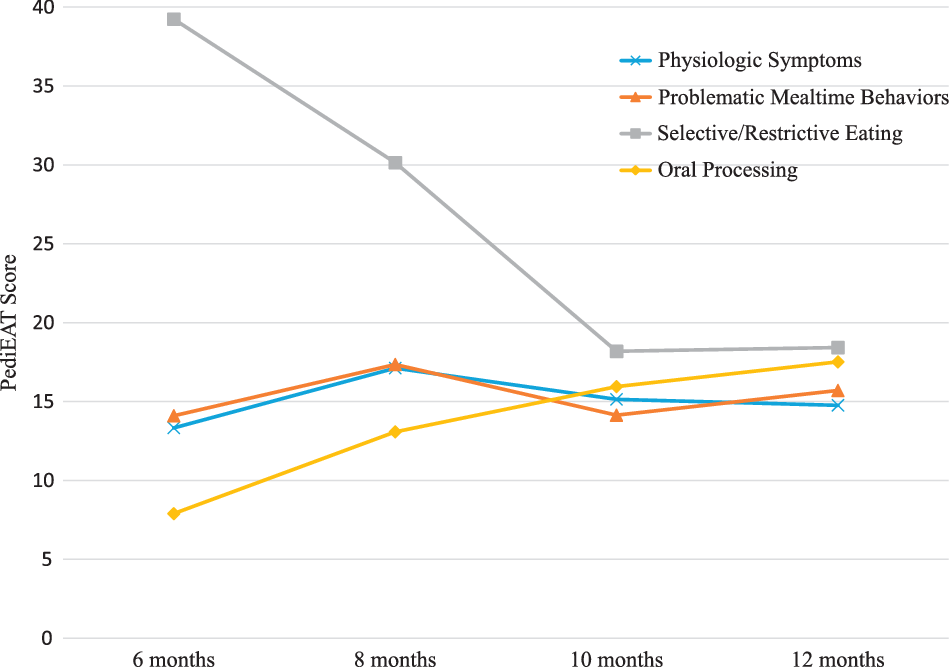
Figure 2. Mean Pediatric Eating Assessment Tool (Pediatric Eating Assessment Tool) subscale scores over time from 6 to 12 months (n = 30). Higher scores indicate more problematic feeding (i.e., worse).
In this sample, at 6 months, 24 of 25 (96%) infants met criteria for any feeding problem. At 8 months, 18 of 27 (67%) infants met criteria for any feeding problem. At 10 months, 16 of 23 (70%), and at 12 months, 9 of 21 (43%) met criteria for any feeding problem (Table 1). There was no significant difference between infants with single and double ventricle physiology for any of the subscales or total scores of the Pediatric Eating Assessment Tool or Child Oral and Motor Proficiency Scale at any of the time points.
Relationships between feeding skills & behaviours
Correlations between the Child Oral and Motor Proficiency Scale (feeding skill) and Pediatric Eating Assessment Tool (feeding behaviour) are presented on Table 2. Child Oral and Motor Proficiency Scale total score and Pediatric Eating Assessment Tool total score were significantly correlated at all time points; however, the relationships between the subscale scores changed over time.
Table 2. Correlations (Spearman’s rho) between feeding skills (ChOMPS) and behaviours (PediEAT) at 6 (n = 21), 8 (n = 26), 10 (n = 21), and 12 (n = 20) months
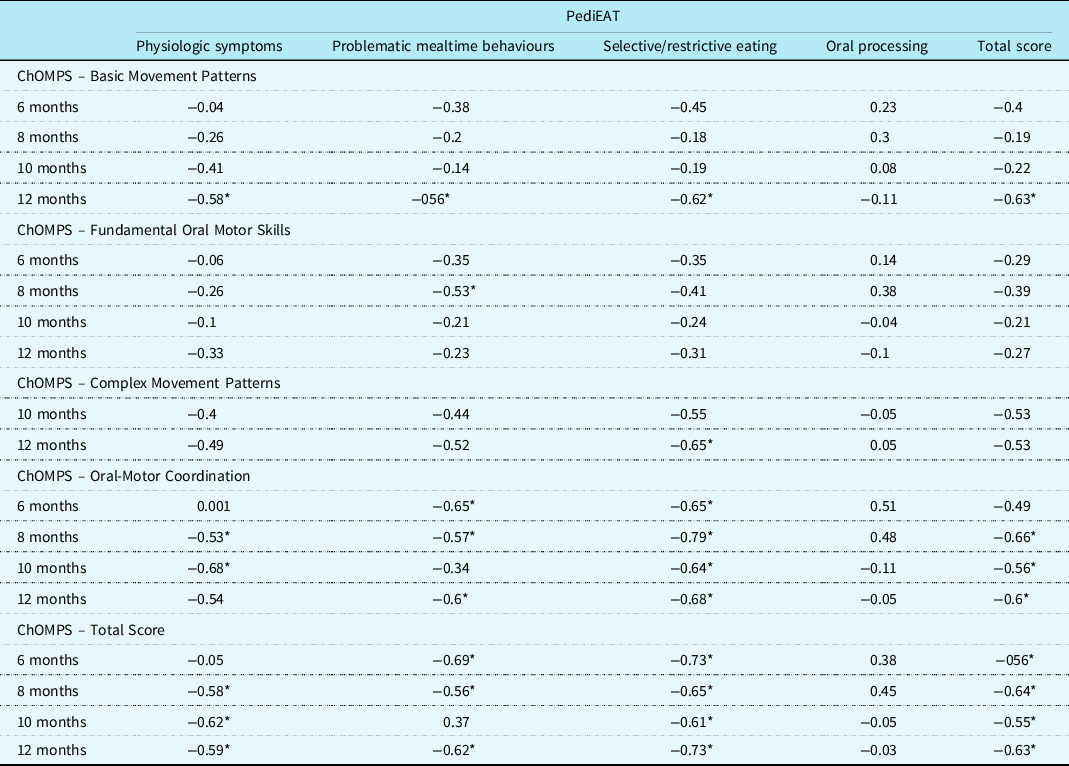
Note. ChOMPS = Child Oral and Motor Proficiency Scale, range of scores 0–126, higher scores indicate more skill (better); PediEAT = Pediatric Eating Assessment Tool, range of scores 0–390, higher scores indicate more symptoms of problematic feeding (i.e., worse). The ChOMPS Complex Movement Patterns subscale is not appropriate for infants aged <9 months, so scores are not reported for that subscale at 6 and 8 months.
* Indicates p < 0.01.
The Child Oral and Motor Proficiency Scale Basic Movement Patterns subscale was not significantly correlated with any Pediatric Eating Assessment Tool subscale or total score at 6, 8, or 10 months. However, at 12 months, there was a statistically significant negative correlation between the Child Oral and Motor Proficiency Scale Basic Movement Patterns subscale and all Pediatric Eating Assessment Tool subscales and total score (except Oral Processing; see Table 2).
The Fundamental Oral Motor Skills subscale of the Child Oral and Motor Proficiency Scale was only significantly correlated with the Problematic Mealtime Behaviors subscale of the Pediatric Eating Assessment Tool at 8 months (rho = −0.53, p < 0.01). The Complex Movement Patterns subscale of the Child Oral and Motor Proficiency Scale was only significantly correlated with the Pediatric Eating Assessment Tool subscale of Selective/Restrictive Eating at 12 months (rho = −0.65, p < 0.01). The Oral-Motor Coordination subscale of the Child Oral and Motor Proficiency Scale was significantly correlated with multiple Pediatric Eating Assessment Tool subscales and total scores at each of the time points (Table 2).
Feeding skills, behaviours, and gastrointestinal tract symptoms
There were no significant relationships between the Child Oral and Motor Proficiency Scale and Infant Gastrointestinal Symptoms Questionnaire at any time point. At 8 months, there was a significant relationship between the Pediatric Eating Assessment Tool Physiologic Symptoms subscale and the Infant Gastrointestinal Symptoms Questionnaire (rho = 0.51; p = 0.009). The full table of correlations is available in Supplementary Table S1.
At 12 months, the Infant Gastroesophageal Reflux Questionnaire-Revised was significantly correlated with the Child Oral and Motor Proficiency Scale subscales for Basic Movement Patterns (rho = −0.69, p = 0.001) and Oral-Motor Coordination (rho = −0.64, p = 0.002). There were significant relationships between the Infant Gastroesophageal Reflux Questionnaire-Revised with the Pediatric Eating Assessment Tool Physiologic Symptoms at 12 months (rho = 0.59, p = .006); Problematic Mealtime Behaviors at 12 months (rho = 0.67, p = 0.001); Selective/Restrictive Eating at 12 months (rho = 0.6, p = 0.006), and Total score at 6 months (rho = 0.64, p = 0.002), 8 months (rho = 0.52, p = 0.006), and 12 months (rho = 0.67, p = 0.001).
Discussion
In this longitudinal study, we examined the feeding skills, behaviours, and gastrointestinal tract symptoms of 33 infants with CHD from 6 months through 1 year of age, when solid foods are typically introduced and when infants are exposed to increasing complexity in tastes and textures of food. Infants with CHD, particularly those who require surgery, are exposed to negative experiences around their face and mouth. The face and mouth are highly innervated. Reference Dall'Orso, Steinweg, Allievi, Edwards, Burdet and Arichi21 Trauma from endotracheal tubes, continuous positive airway pressure devices, nasogastric or orogastric tubes, and tape on the face to secure tubes in place may alter the development of sensation and motor innervation of the face and mouth, Reference Dall'Orso, Steinweg, Allievi, Edwards, Burdet and Arichi21 placing these infants at risk for a variety of feeding-related problems. Additionally, feeding experiences are often disrupted in the early postnatal period and these infants may miss early opportunities for establishing oral feeding skills Reference Goldstein, Watkins, Lowery and Yu4,Reference Toms, Jackson, Dabal, Reebals and Alten22,Reference Jones, Desai and Fogel23 and parent–child bonding, Reference Tesson, Butow, Marshall, Fonagy and Kasparian24 which may have long-term impacts on their feeding development.
In this study, we found that, while these infants showed improvement in their eating, drinking, and related feeding skills from 6 to 12 months, many of these infants were delayed in their acquisition of skills compared to healthy infants. Infants with CHD exhibited delays in skills, such as bringing a piece of food to their mouth; using their tongue to move food in their mouth; drinking from a straw; using a filled spoon or fork to bring food to their mouth; or being able to drink thin liquids without gagging, coughing, or choking. Similarly, many of these infants exhibited feeding behaviours that were outside the range of typical feeding, and there was minimal improvement over time. Infants with CHD displayed more problematic behaviours than their healthy peers, such as avoiding or refusing to eat, showing more stress during meals, coughing during or after eating, or preferring smooth foods. These findings suggest that problematic feeding behaviours do not dramatically improve with increasing age in this population. These findings are consistent with another study that found that children up to 7 years of age with CHD had more symptoms of problematic feeding than their healthy peers. Reference Pados7 Our findings support the need for additional research with larger sample sizes in order to develop and test interventions targeted at the specific feeding issues of this population of infants.
In infants with CHD who are struggling with feeding, our data would suggest that early implementation of feeding support services is appropriate and that these infants do not “grow out” of their feeding difficulties. While many of these infants qualify for early intervention services, proactive involvement of feeding specialists in the care of infants born with CHD and/or frequent assessment of developing skills is needed to support these infants in the first year of life and beyond. Feeding specialists, typically speech-language pathologists/therapists or occupational therapists, can help to identify safe oral feeding strategies, appropriate interventions for sensory differences or motor deficits, and strategies to build the necessary skills for more complex feeding and speech.
As expected, feeding skills and feeding behaviours were related, with the Child Oral and Motor Proficiency Scale and Pediatric Eating Assessment Tool total scores being highly correlated at all time points (i.e., lower feeding skill was associated with more problems with feeding behaviour). However, these tools measured unique aspects of feeding. Measurement of both feeding skill and feeding behaviour is necessary for a full understanding of a child’s feeding and to ensure that interventions are targeted appropriately at the underlying problem. For example, an infant who lacks the oral motor skills to manage more complex solid foods that require chewing may display feeding behaviours such as spitting out food or a strong preference for smooth foods that do not require chewing. In this case, it is critical to assess both feeding skill and behaviour to identify that the poor motor skills are likely the underlying cause of the feeding behaviours, and interventions must first target skill development before addressing the feeding behaviours. If interventions first address the behaviour by exposing the infant to more complex foods without building appropriate oral motor skills, the infant may choke and/or gag, which is likely to worsen feeding behaviours. On the other hand, in an infant with appropriate oral motor skills who has developed a negative association with oral feeding because of trauma to the face and mouth, oral motor skill development techniques will not address the underlying problem and, in some cases, may even contribute to the child’s negative oral experiences.
In this sample, we found limited relationships between feeding skills and symptoms of gastrointestinal tract dysfunction, that is, gastrointestinal distress or gastroesophageal reflux. However, we did find that symptoms of problematic feeding behaviours were related to both gastrointestinal distress and gastroesophageal reflux. Further understanding of the relationships between problematic feeding behaviours and gastrointestinal tract symptoms in infants with CHD is necessary for developing appropriate interventions and care plans.
Strengths, limitations, & future directions
The main limitation of this study is a small sample size with further limitations in the number of infants for whom we have complete data at all time points. However, a strength is that we were able to follow these infants from neonatal hospitalisation through 1 year of age. Given the sample size, we were not able to analyse the data by specific diagnosis. Further research with larger sample sizes, representing infants cared for at different institutions and with different types of CHD, will be important to better understand development of feeding skill and feeding behaviour in infants with CHD. Future studies with larger sample sizes should take into account feeding-related therapies and involvement of feeding-related specialists. Additionally, since these data were entirely parent-reported, future research with clinical observation of feeding skills and behaviours and objective assessment of swallowing function and gastroesophageal reflux in this population would add significantly to our understanding of feeding-related difficulties.
Conclusion
In this study, we found that infants with CHD commonly have delayed acquisition of feeding skills and problematic feeding behaviours at 6 to 12 months of age. While feeding skills and feeding behaviours are related, they each contribute uniquely to overall feeding success. Assessment of both skill and behaviour is necessary for targeted implementation of interventions. Symptoms of gastrointestinal distress and gastroesophageal reflux are also related to symptoms of problematic feeding, particularly feeding behaviours. Early and frequent assessment, targeted interventions, and early involvement of feeding specialists may help to support these infants as they develop through the first year of life and beyond.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951122003298
Acknowledgements
The authors would like to acknowledge the families who participated in this study. They would also like to acknowledge Sandra Solove for her excellent work in managing the project.
Financial support
This work was supported by National Institutes of Health (T.M.H., grant number R15NR017092), the Dhillon Jordan Shah Innovation Fund in CHD (B.P.), and Boston College Vice Provost for Research Faculty Research Funds (B.P.).
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation (US Department of Health & Human Services, Office for Human Research Protections) and with the Helsinki Declaration of 1975, as revised in 2008, and have been approved by the institutional committee (Nationwide Children’s Hospital).







