Bleeding is a known risk for children undergoing cardiac surgery involving cardiopulmonary bypass and is related to hemodilution, haemostatic defects such as platelet dysfunction, inflammation, and hypothermia. Reference Paparella, Brister and Buchanan1–Reference Despotis, Gravlee, Filos and Levy6 Critically ill children with bleeding have worse clinical outcomes including higher exposure to blood component transfusions, increased length of intensive care stays, longer requirement for vasoactive medications, and increased mortality. Reference Dalton, Garcia-Filion and Holubkov7–Reference White, Fredericks, Mannarino, Janofsky and Faustino9 Several studies have described the epidemiology of bleeding with children undergoing cardiopulmonary bypass; however, there has been no standard definition of bleeding used throughout these studies and each uses their own local definition, making the data difficult to interpret and integrate for care across varied populations of children with CHD. Reference Pinto, Shabanova and Li10–Reference Nellis, Levasseur and Stribling13
In order to provide a standard definition across all critically ill children, the Bleeding Assessment Scale in critically Ill Children (BASIC) has recently been developed from expert physician consensus. Reference Nellis, Tucci and Lacroix14 The BASIC definition includes quantifiable blood loss, perturbations in physiologic variables specific to children, and complications of bleeding affecting organ dysfunction in its definition rather than interventions, such as the need for blood transfusions. Though paediatric cardiac intensivists were involved in the development of the consensus definition, BASIC has yet to be applied specifically in a population of children undergoing cardiac surgery with cardiopulmonary bypass.
We sought to describe the epidemiology of severe bleeding up to 24 hours post-operatively in critically ill children who have undergone cardiopulmonary bypass surgery using the BASIC definition and to identify the association between severe bleeding and clinical outcomes.
Materials and methods
We conducted a retrospective single centre cohort study. The Institutional Review Board at Weill Cornell Medicine approved this study (protocol #1811019782). We included patients aged 1 day to 18 years undergoing any cardiac surgery involving cardiopulmonary bypass admitted to the paediatric ICU at New York Presbyterian-Weill Cornell Medicine (NYP-WCM) from January 2015 to December 2020. Of note, the paediatric ICU was closed to paediatric patients from March to May of 2020 due to the Coronavirus-19 pandemic. Patients were excluded if there was a known limitation of care at enrollment, pre-existing bleeding disorder, or required extracorporeal membrane oxygenation. All patients were managed post-operatively in the mixed paediatric ICU at NYP-WCM.
In general, the cardiopulmonary bypass circuit was primed with red blood cells and plasma if the patient was <3 kg, red blood cells if the patient was ≥3 kg but <10 kg and crystalloid if ≥10 kg. Blood products used to prime the circuit were not included in the volume of intra-operative transfusions. Prior to cannulation, each patient received heparin (400 units/kg) which was reversed with protamine following separation from cardiopulmonary bypass (protocolised dosing until activated clotting time returned to baseline prior to cardiopulmonary bypass). There were no standard transfusion protocols used and any bleeding intervention was determined at the discretion of the cardiology, cardiothoracic, and intensive care teams. No viscoelastic testing was used to guide haemostatic interventions.
Study variables obtained from the electronic medical record were patient demographics, intraoperative data including blood products administered, cardiopulmonary bypass and aortic cross clamp time, and surgical complexity. Surgical complexity was categorised using the Risk Adjustment for Congenital Heart Surgery (RACHS) scoring. Reference Jenkins, Gauvreau, Newburger, Spray, Moller and Iezzoni15 Post-operative laboratory values including haemoglobin, platelet count, fibrinogen, prothrombin time, activated partial thromboplastin and international normalised ratio were obtained upon admission to the paediatric ICU and assayed once. Intraoperative and post-operative medication and blood product administration were recorded.
Clinical outcomes included paediatric ICU-free days, mechanical ventilation-free days, and mortality. Each of these were calculated from the first 28 days in the paediatric ICU. The data were managed and secured by using the Weill Cornell Medicine Research Electronic Data Capture (REDCap) application.
Severe bleeding was determined by using the Bleeding Assessment Scale in Critically Ill Children (BASIC) definition as shown in Supplemental Figure 1. The classification of bleeding was determined by two investigators based on chart review. Discrepancies in classification were resolved by the third reviewer. The investigators were not blinded to the clinical outcomes.
Statistical analysis
Demographic and clinical characteristics were described as n (%) or median and interquartile range, as appropriate. Children who had a severe bleeding event during the first 24 hours post-operatively were compared to children who did not have severe bleeding by Chi-square/Fisher’s Exact tests or Wilcoxon rank-sum tests. Receiver operator curves were constructed to evaluate the ability of laboratory testing to predict severe bleeding. Linear regression was performed to assess the association between severe bleeding, covariates, and clinical outcomes. Two-sided p values below 0.05 were considered significant. All analyses were conducted using SPSS version 25 (IBM Corp, Armonk, NY).
Results
Three hundred fifty-six paediatric patients were included in the study. Of these enrolled patients, 211 (59%) were male and the median (IQR) age was 2.1 (0.5–8.1) years. Twenty-seven (7%) were neonates. The majority of patients (70%, 250/356) were undergoing initial repair, and surgical complexity was categorised as RACHS 2 (48%, 170/356) and RACHS 3 (34%, 122/356) followed by RACHS 1 (15%, 52/356) and RACHS 4 (3%, 12/356). None of the surgeries included were considered RACHS 5 or 6. The median age of the patients by RACHS score is reported in the Supplemental Data.
Severe bleeding was observed in 16% (57/356) of enrolled patients. The rate of severe bleeding in the neonates was 22% (6/27). The patients qualified for severe bleeding based on the following BASIC criteria (which were not mutually exclusive): 84% (48/57) had quantifiable bleeding >5 ml/kg/hr for more than 1 hour, 35% (20/57) had bleeding that led to haemodynamic instability, 26% (15/57) had bleeding that led to a drop in haemoglobin by >20% within 24 hours, and 7% (4/57) had bleeding that led to new organ dysfunction. The criteria met by the nine patients who did not have >5 ml/kg/hr of quantifiable blood loss are described in the Supplementary Data. No patients had intraspinal, intraarticular, or intraocular bleeding. There were no fatal bleeding events.
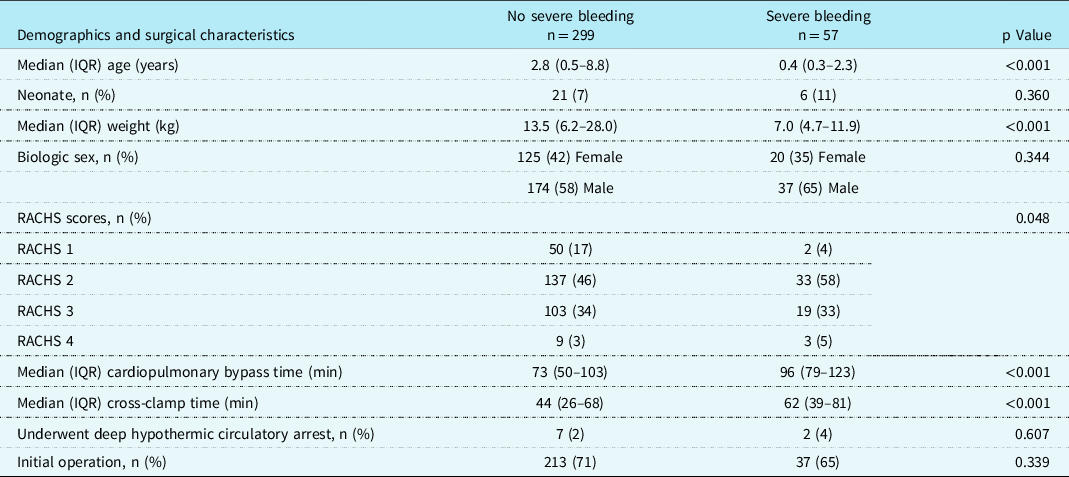
The demographics and surgical variables of those with severe bleeding compared to those without severe bleeding are described in Table 1. Severe bleeding was observed more frequently in younger (median [IQR] age 7.0 [4.7,11.9] months in severe bleeding versus 34.0 [5,105] months without severe bleeding, p < 0.001) and smaller (p < 0.001) children and those with longer bypass (median [IQR] 96 [79,123] minutes in severe bleeding versus 73 [50,103] minutes without, p < 0.001) and cross-clamp (median [IQR] 62 [39,81] minutes in severe bleeding versus 44 [26,68] minutes without, p < 0.001) times with higher surgical complexity (p = 0.048). Biologic sex (p = 0.344), deep hypothermic circulatory arrest (p = 0.607), and initial operation for the cardiac defect (p = 0.339) were not significantly associated with severe bleeding.
Table 1. Demographics and surgical characteristics of children with and without severe bleeding undergoing cardiac surgery involving cardiopulmonary bypass.
The blood components received intra-operatively by all patients were as follows: red blood cells 141/356 (40%), cell saver 180/356 (51%), platelets 128/356 (36%), plasma 30/356 (8%), and cryoprecipitate 56/356 (15%). The blood components received post-operatively in the paediatric ICU by all patients were as follows: red blood cells 30/356 (8%), platelets 48/356 (13%), plasma 18/356 (5%), and cryoprecipitate 24/356 (7%). Thirty-seven (10%) of the patients received no blood products. Of the 57 patients with severe bleeding, 33% (19/57) received red blood cells transfusions in the paediatric ICU, 49% (28/57) received platelets, 18% (10/57) received plasma, and 32% (18/57) received cryoprecipitate. Two (4%) of the patients with severe bleeding received no blood products. In patients with severe bleeding who received red blood cells, the median (IQR) dose in the paediatric ICU was 9.8 (8.9–13.0) ml/kg. In patients with severe bleeding who received platelets, the median (IQR) dose was 10.0 (9.0–11.7) ml/kg. In patients with severe bleeding who received plasma, the median (IQR) dose was 13.7 (10.2–18.8) ml/kg. In patients with severe bleeding who received cryoprecipitate, the median (IQR) dose was 4.3 (2.7–5.3) ml/kg. Figure 1 describes the doses of all blood products administered during the operative procedure and post-operatively broken down by bleeding groups. Those with severe bleeding post-operatively received significantly more red blood cells (p < 0.001), platelets (p < 0.001), plasma (p =<0.001), less cell saver (p = 0.001), but not cryoprecipitate (p = 0.315) in the operating room as compared to those without severe bleeding. Similarly, post-operatively, those with severe bleeding received more red blood cells, platelets, plasma, and cryoprecipitate (all p-values < 0.001).
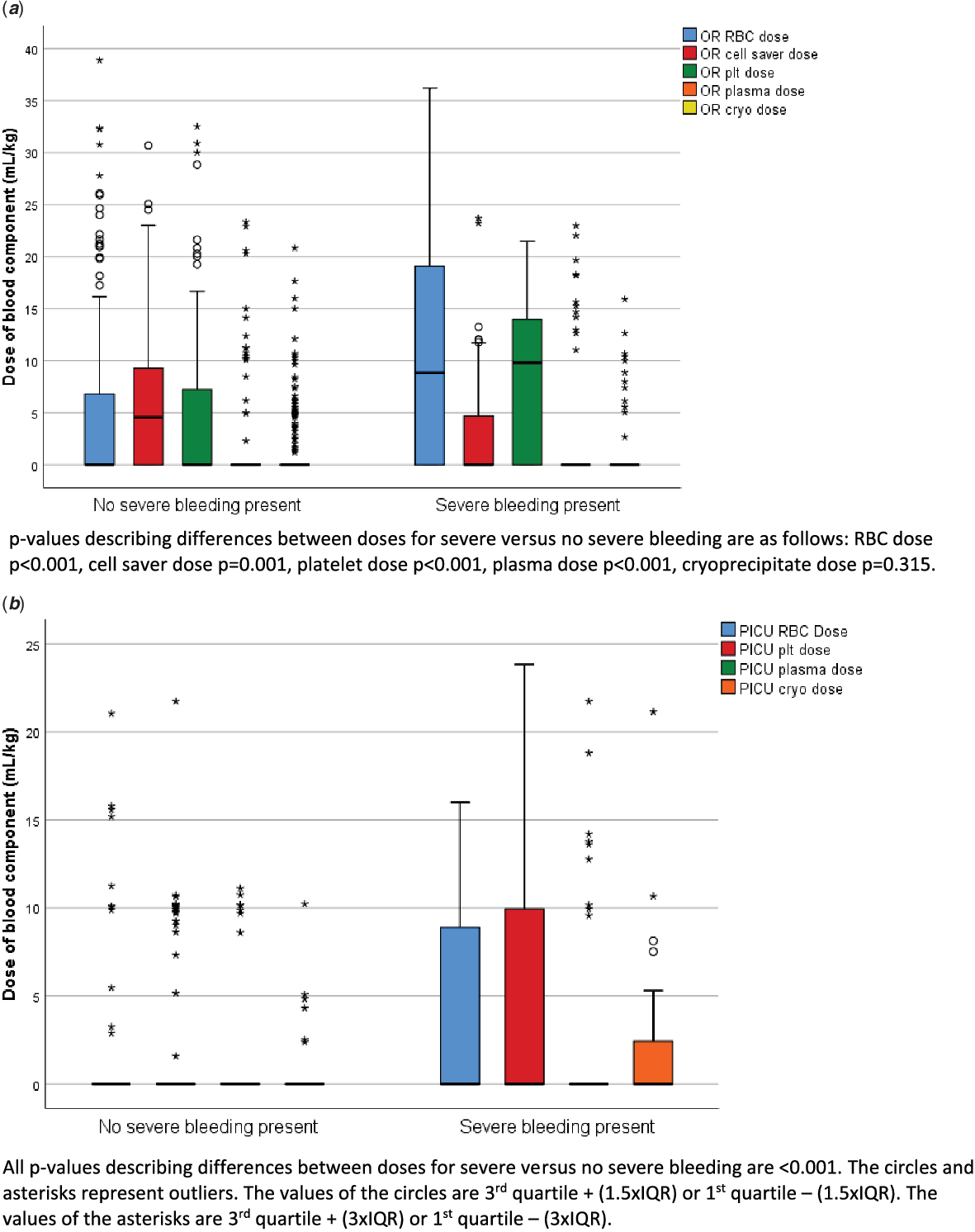
Figure 1. Blood component administration intra-operatively ( a ) and post-operatively ( b ) between those with severe bleeding post-operatively and without severe bleeding post-operatively. The “0” and “*” represent outliers as reported within SPSS.
Intra-operatively, nearly all patients received a haemostatic medication including: aminocaproic acid (75%, 269/356), tranexamic acid (17%, 65/356), and desmopressin (4%, 10/356). No patients received activated Factor VII in the operating room or post-operatively. There were no differences in the receipt of aminocaproic acid (p = 0.754), tranexamic acid (p = 0.368), or desmopressin (p = 0.599) between those with post-operative severe bleeding and those without. No patients required chest exploration to control bleeding.
Table 2 illustrates the comparison in laboratory values at paediatric ICU admission in those with severe bleeding versus those without severe bleeding. There were no significant differences in platelet count (p = 0.790) between those with severe bleeding and those without. However, there were significant differences in activated partial thromboplastin time (aPTT) (p < 0.001), prothrombin time (PT) (p = 0.039), international normalised ratio (p = 0.037), and fibrinogen (p = 0.019). Receiver-operator characteristic curves for the ability of laboratory tests to predict severe bleeding are demonstrated in Figure 2. The area under the curve with 95% CI were as follows: PT 0.604 (0.524–0.684), aPTT 0.674 (0.600–0.747), international normalised ratio 0.596 (0.514–0.678), platelet count 0.605 (0.500–0.710), and fibrinogen 0.669 (0.557–0.780); all area under the curve values demonstrated poor discriminatory value. No viscoelastic testing was performed.
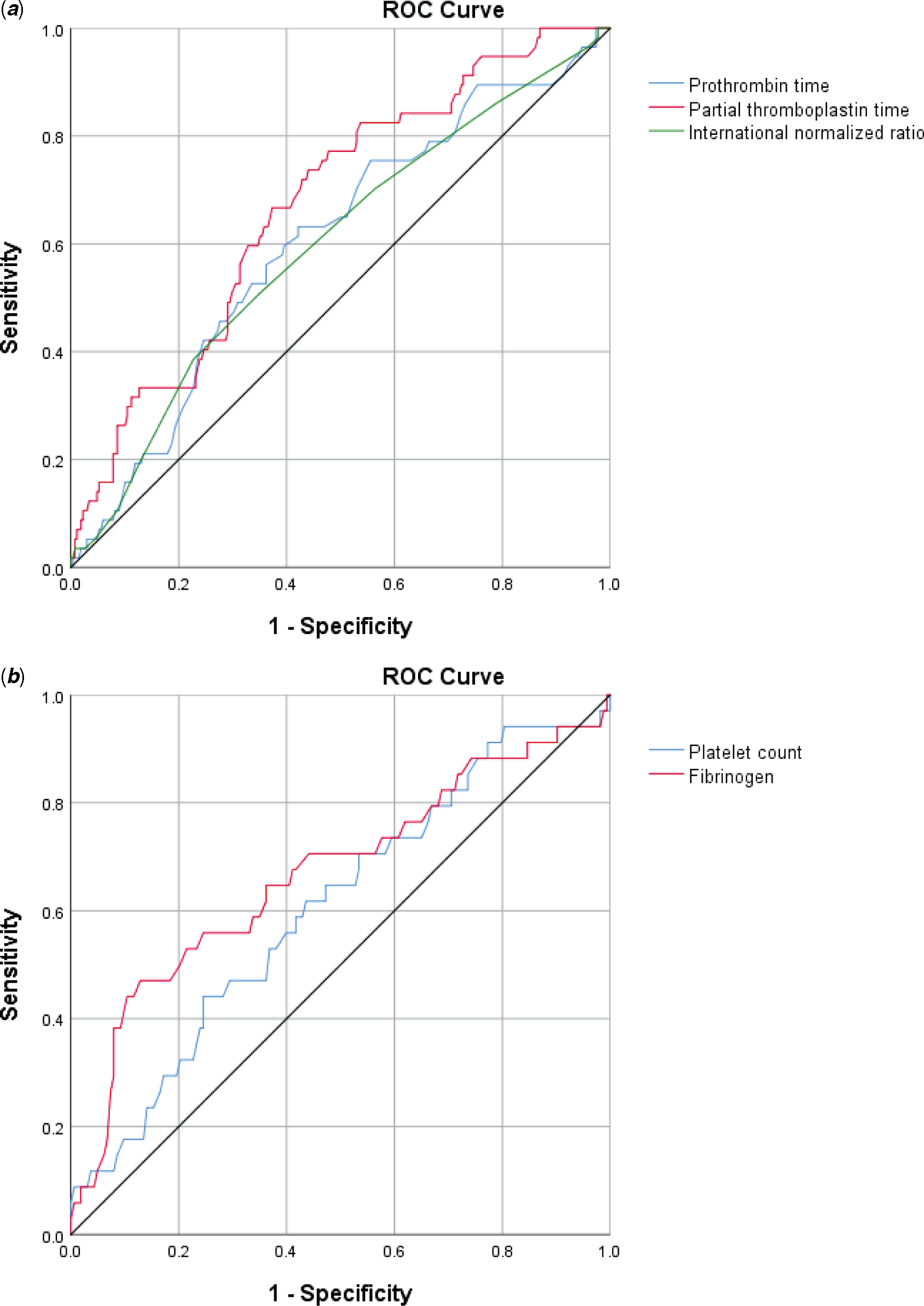
Figure 2. Receiver operator characteristic curves for the ability of laboratory values at paediatric ICU admission to predict severe bleeding pos-operatively. ( a ) Highest PT, aPTT, and INR and ( b ) Lowest platelet count and fibrinogen.
Table 2. Laboratory values for children with and without severe bleeding undergoing cardiac surgery involving cardiopulmonary bypass.
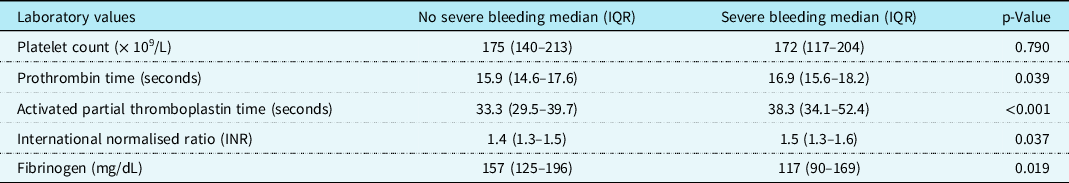
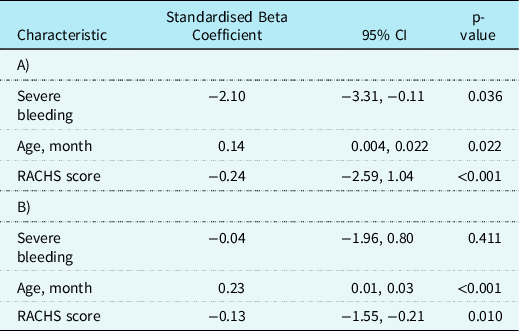
Table 3 presents the associated clinical outcomes. No patients included in the study died. The median (IQR) number of paediatric ICU-free days for those with severe bleeding was 22 (18–23) and for those without severe bleeding was 23 (21–24) (p = 0.010). The median (IQR) number of mechanical ventilation-free days for those with severe bleeding was 26 (24–27) and for those without severe bleeding was 27 (26–28) (p = 0.013). When adjusted for age and RACH score, severe bleeding continued to be associated with fewer paediatric ICU-free days (β coefficient −2.1, 95% CI −3.31 to −0.11, p = 0.036) but not associated with mechanical ventilation-free days (β coefficient −0.04, 95% CI −1.96 to 0.80, p = 0.411).
Table 3. Clinical outcomes for children with and without severe bleeding undergoing cardiac surgery involving cardiopulmonary bypass. (A) Association between severe bleeding and paediatric ICU-free days and (B) association between severe bleeding and mechanical ventilation-free days.
Discussion
Bleeding is a significant concern in paediatric patients undergoing cardiac surgery involving cardiopulmonary bypass, with 16% of these children having a severe bleeding episode within the first 24 hours post-operatively based upon the BASIC definition. Several patient and surgical characteristics were associated with severe post-operative bleeding including younger age, smaller weight, higher surgical complexity, longer cardiopulmonary bypass times, and longer aortic cross clamp times. No laboratory values obtained on admission to the paediatric ICU had good discriminatory value to predict severe post-operative bleeding. Patients with severe bleeding had worse clinical outcomes including time of mechanical ventilation and length of stay in the paediatric ICU.
This study represents the first to apply the BASIC definition to characterise post-operative bleeding in children. Previous studies have used varying definitions of bleeding in this population. In a scoping review contained within an observational cohort, Bercowitz reported over 20 definitions of bleeding applied to children following cardiopulmonary bypass (CPB). Reference Bercovitz, Shewmake and Newman12 This resulted in a huge variation in the estimation of the prevalence of bleeding, as well as difficulty comparing other results across studies (such as the ability of laboratory tests to predict bleeding). By applying a definition developed for all critically ill children based on physiologic parameters and sites of bleeding particular to paediatrics and not based on subjective interventions (such as the prescription of a RBC transfusion), the prevalence of bleeding in children following CPB can be standardised and compared to other groups of critically ill children. Using the BASIC definition, our finding that severe bleeding in children following CPB is associated with poorer clinical outcomes mirrors that reported in a heterogenous prospective cohort of critically ill children, Reference Sequeira, Nellis and Karam16 as well as those with an underling oncologic diagnosis. Reference Romano, Martinez, Levasseur, Killinger, Karam and Nellis17
Previous studies, using a variety of definitions, have reported the rate of severe bleeding following cardiopulmonary bypass in children to range from 3 to 42%. Reference Bercovitz, Shewmake and Newman12 Various studies have concluded that younger age is associated with an increased risk of bleeding. Reference Pinto, Shabanova and Li10,Reference Williams, Bratton and Ramamoorthy18,Reference Williams, Bratton, Riley and Ramamoorthy19 This is replicated in our cohort. These findings may be related to the maturing haemostatic system of the infant in which there are lower levels of coagulation factors (though these are often balanced by lower levels of anti-coagulation factors), as well as CPB circuit priming leading to coagulation factor dilution. Reference Andrew, Paes and Milner20,Reference Guzzetta, Allen, Wilson, Foster, Ehrlich and Miller21 Weight appeared to be inversely related to clinically relevant bleeding which is in concordance with previous findings of children weighing less than 8 kg having higher rates of bleeding after undergoing CPB. Reference Miller, Mochizuki and Levy22 We found that there was no difference in bleeding when comparing biologic sex, and whether this was the patient’s initial operation for the cardiac defect.
We did not identify any standard laboratory values that were predictive of severe bleeding which is consistent with recently reported results from a large systematic review. Reference Delaney, Karam and Lieberman23 Smaller reports have examined the role of fibrinogen and thrombocytopenia. Low fibrinogen has been previously reported with increased chest tube bleeding in both children following CPB. Reference Nellis, Dalton and Karam24–Reference Pekelharing, Furck, Banya, Macrae and Davidson26 Evidence suggests that low levels of fibrinogen are the earliest haemostatic derangement during CPB and contribute to post-operative bleeding. Reference Levy, Szlam, Tanaka and Sniecienski27–Reference Faraoni, Willems, Savan, Demanet, De Ville and Van der Linden29 Recent trials have evaluated the efficacy of cryoprecipitate versus fibrinogen concentrate replacement in these children. Reference Downey, Andrews and Hedlin30 In addition, prior studies have reported an association between thrombocytopenia and increased bleeding in children following CPB. Reference Williams, Bratton and Ramamoorthy18,Reference Williams, Bratton, Riley and Ramamoorthy19 Although the platelet count has been noted to drop in children following CPB, rarely does thrombocytopenia develop (<100 × 109 cells/L), but rather platelet dysfunction develops. Reference Bønding Andreasen, Hvas and Ravn2 While the exact mechanisms of platelet dysfunction are not fully understood, it is thought to be related to blood contact with the CPB circuit, hemodilution, and hypothermia. Reference Tempe and Virmani31,Reference Chan, Leaker and Burrows32
Because standard laboratory assays do not typically predict bleeding, as confirmed in our cohort, whole blood assays, such as viscoelastic testing, should be considered in this patient population. Several studies have reported decreased bleeding and/or blood product usage in children undergoing CPB surgeries when viscoelastic testing is used. Reference Nakayama, Nakajima and Tanaka3,Reference Mendeloff, Glenn and Tavakolian33–Reference Kane, Woodward, Husain and Frei-Jones35 Based on this literature, the Transfusion Anemia eXpert Initiative – Control/Avoidance of Bleeding (TAXI-CAB) suggested that viscoelastic testing should be considered in the paediatric ICU post-operatively (level 2B recommendation). Reference Cholette, Muszynski and Ibla36
We found that those with severe bleeding received more red blood cell, platelet, and plasma transfusions both intra and post-operatively. Prior studies have documented the increased rate of haemostatic transfusions among bleeding in children following CPB. Reference Closson, Mauer and Stock8,Reference White, Fredericks, Mannarino, Janofsky and Faustino9 While red blood cells and cell saver were the most commonly transfused products in our cohort, which is similar to reports from much larger observational databases, our rates of transfusion were lower. Reference Hanson, Karam and Birch37 This may be a reflection of the surgical complexity of our cohort as compared to others reported in the literature. The use of a transfusion algorithm may decrease the number of transfusions even further. Reference Cholette, Muszynski and Ibla36,Reference Whitney, Daves and Hughes38
There are several strengths to this study. The bleeding definition used was developed by a diverse group of experts in haemostasis, led by the senior author, and included cardiac intensivists, haematologists, anaesthesiologists, surgeons, and transfusion medicine specialists. The definition was formulated through a Delphi process and not simply derived for this study. The cohort was accrued from a single centre with no turnover of surgical staff during the reported time period reported, therefore there are fewer variables related to surgical technique that may contribute to the risk of bleeding and confound the analysis of associated laboratory data. We reported all the haemostatic agents received.
However, there are several limitations. First, the study was retrospective and only involved one centre and therefore may not be generalisable. Our centre has a relatively low number of cases a year (80–100) with few neonatal repairs and therefore the results, particularly the rate of severe bleeding, may not be generalisable. Transfusions and use of haemostatic medications were not protocolised. There was also an absence of higher risk CHDs (RACHS 5 and 6). Given the higher complexity of these surgeries along with likely more medically complex patients, the rates of severe bleeding may be higher than reported here. Given the degree of hemodilution and lower levels of vitamin K-dependent clotting factors in neonates, the laboratory values assayed in these children may be a reflection of insufficient treatment and not severity of disease. Future studies should also report separately the blood product exposure in the CPB prime. In addition, some subjectivity exists due to the BASIC definition relying on the attribution of clinical or laboratory changes to bleeding alone, and not other pathophysiologic reasons for these changes. We attempted to account for this by having two reviewers adjudicate each bleeding episode. The true prevalence of bleeding may be more accurately described in a prospective cohort. Intraspinal, intra-articular, and intra-ocular bleeding did not occur in this cohort and could be adapted in future consensus definitions. By using a definition of bleeding that incorporates scores of organ dysfunction, the score may be influenced by treatment and may not accurately reflect the severity of disease.
Conclusion
Using the BASIC definition, 16% of children undergoing CPB with RACHS scores of 1–4 have severe bleeding within 24 hours of surgery. Younger age, lower weight, and longer CPB and cross clamp times were associated with a higher risk of severe bleeding. No laboratory assays obtained on admission were able to predict post-operative severe bleeding. Prospective studies are needed to understand the mechanisms of severe bleeding so that we can have improved prophylactic and therapeutic strategies.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951122003493
Financial support
Research reported in this publication was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002384. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest
None
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation in the United States and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional review board at Weill Cornell Medicine.








