Cardiac involvement in patients with COVID-19 has been extensively described in the adult literature with evidence suggesting that elevations in cardiac biomarkers such as high-sensitivity troponin T and N-terminal pro-brain type natriuretic peptide are associated with more severe disease including mechanical ventilation and inotropic support. Reference Goyal, Choi and Pinheiro1–Reference Wang, Hu and Hu7 Though cardiac findings have been well described in cases of multi-system inflammatory syndrome in children, Reference Feldstein, Rose and Horwitz8,Reference Cheung, Zachariah and Gorelik9 similarly robust data are not available for children with positive COVID-19 tests who do not have multi-system inflammatory syndrome in children. In this study, we describe the cardiac findings in children with acute COVID-19 who received care at a large tertiary care children’s hospital in New York City, the initial epicentre in the United States of America, and describe outcomes in this population. Ultimately, we hope to use this information to standardise cardiac workup and monitoring in children with acute presentations of COVID-19 infection and guide early management decisions.
Methods
Study design and population
We performed a retrospective cohort study of patients under age 21 who presented to Morgan Stanley Children’s Hospital emergency department or paediatric inpatient units and tested positive for SARS-CoV-2 on real-time polymerase chain reaction testing between 11 March 2020 (first positive paediatric case in our institution) and 17 May 2020. This period includes all patients from the start of the COVID-19 pandemic at our institution through approximately 1 month past our initial institutional peak caseload. Patients with complex CHD, cardiomyopathy, heart transplant, or multi-system inflammatory syndrome in children were excluded. This study was approved by the Columbia University Irving Medical Center Institutional Review Board with waiver of informed consent.
Diagnosis/testing
Samples for SARS-CoV-2 were collected via nasopharyngeal swabs and analysed via real-time polymerase chain reaction. Indeterminate polymerase chain reaction tests were considered positive as per institutional protocol. All diagnostic testing was done for clinical purposes and no additional testing was obtained solely for the purposes of this research. Testing availability, including SARS-CoV-2 polymerase chain reaction and cardiac serology, was limited early in the pandemic response, so patients who presented earlier may not have been tested, therefore may have been inadvertently excluded. Multi-system inflammatory syndrome in children was defined as per Centers for Disease Control case definition. 10 Comorbidities were determined based on documentation in the electronic medical record.
Data collection
Patients were identified by a search of the electronic medical record for all cases with positive SARS-CoV-2 polymerase chain reaction during the study period and entered into an electronic database.
Definitions
Elevated cardiac biomarkers were defined as any elevated high-sensitivity troponin T >14 ng/dL in females and >22 ng/dL in males (lab reference range) or any N-terminal pro-brain type natriuretic peptide > 450 pg/mL in children under age 4 or >300 pg/mL in patients 4 years of age and older. Reference Ross11 Patients who did not have either high-sensitivity troponin T or N-terminal pro-brain type natriuretic peptide measured were not included in the cardiac biomarker analyses. Left ventricular dysfunction was defined as left ventricular ejection fraction <55% on any echocardiogram. Reference Lopez, Colan and Frommelt12,Reference Lang, Bierig and Devereux13 Baseline lab value was defined as values drawn at time of presentation and peak value was defined as the highest value obtained during admission during the study period. Lab values below the limits of detection (high-sensitivity troponin T <6 and N-terminal pro-brain type natriuretic peptide <5) were recorded as values just below the lower limit of detection. Lab values above the threshold for reporting (C-reactive protein > 300, N-terminal pro-brain type natriuretic peptide >70,000) were reported as values just over the upper reporting limit. For example, high-sensitivity troponin T values <6 were recorded as a value of 5. C-reactive protein > 300 was recorded as 301. Weight categories were determined by weight-for-height percentiles for patients under age 2, 14 BMI percentile for patients aged 2–20 15,16 , and BMI for patients aged 20–21, with obesity staging assigned as per established standards. 17 Severe outcome was defined as treatment with advanced respiratory support (non-invasive positive pressure ventilation or invasive mechanical ventilation above baseline), treatment with inotropic or vasoactive agents, or death from any cause.
Electrocardiogram and echocardiogram analysis
Electrocardiograms for all patients were reviewed by a single experienced cardiologist (TJS), who also calculated the corrected QT intervals manually. All baseline ECGs were analysed. Echocardiographic data were extracted from the filed reports.
Statistics
Clinical and demographic variables were reported using standard summary statistics including medians and interquartile ranges for non-parametric data. Due to the small number of patients with elevated cardiac biomarkers, comparative statistics were not reported.
Results
Study population
During the study period, 103 patients had positive SARS-CoV-2 polymerase chain reaction. Of those, 17 were excluded for multi-system inflammatory syndrome in children, 4 were excluded for cardiomyopathy, and 2 for complex CHD including 1 with repaired Taussig–Bing anomaly and 1 with double outlet right ventricle who was in the immediate post-operative period following Fontan surgery. Eighty patients were included in the final analyses, 31/80 (39%) of whom were female, and the median age was 12.5 years (interquartile range 1.9–17.5). The median time from symptom onset to hospital presentation was 2 days (interquartile range 0–5) and from presentation to discharge was 3 days (interquartile range 1–8.5). There were 16/80 (20%) admissions to the paediatric intensive care unit, 41/80 (51%) to the medical ward, and 23/80 (29%) were discharged from the emergency department.
Comorbidities were present in 30/80 (38%) patients and included asthma, diabetes mellitus, chronic renal disease, oncologic disorder, sickle cell disease, and genetic syndromes, among others. One patient had recently undergone orthotopic liver transplant. Two patients had sickle cell disease. Genetic disorders were present in five patients including one with newly diagnosed Brugada syndrome with genetic testing notable for a variant of unknown significance in the SCN5A gene Reference Hyun Choi, Silver, Fremed and Liberman18 (Table 1).
Table 1. Patient characteristics and testing
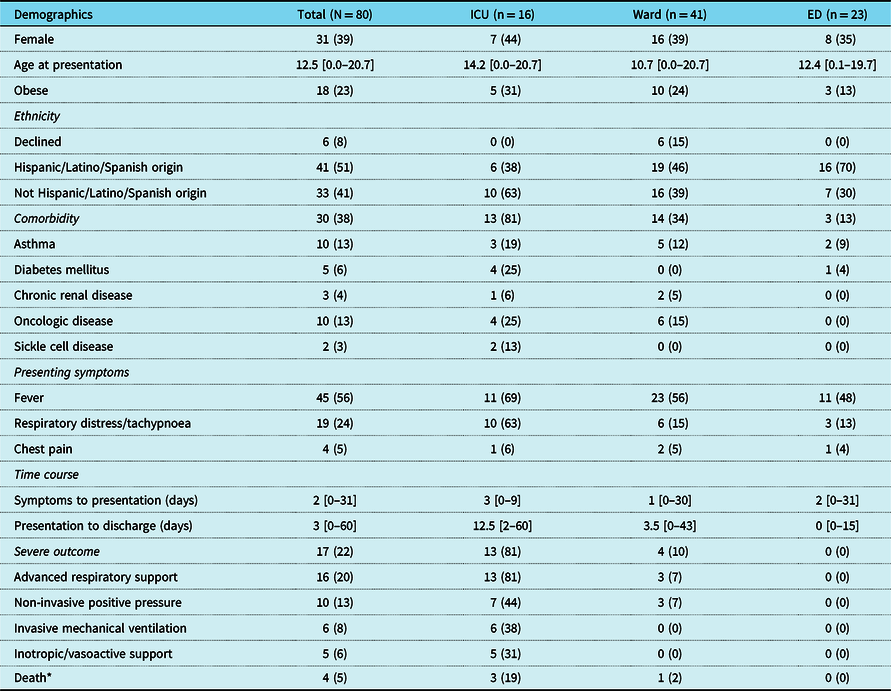
Values represent N (%) for categorical variables and median [range] for continuous variables.
* Two deaths occurred after study period: one directly attributed to COVID-19 complications and the other due to complications from oncologic disease.
Severe outcomes
Severe outcomes were experienced by 17/80 (22%) patients overall and included 16/80 (20%) patients treated with advanced respiratory support, 10/80 (13%) treated with non-invasive positive pressure ventilation, 6/80 (8%) treated with invasive mechanical ventilation, 5/80 (6%) supported with inotropic or vasoactive agents, and 4/80 (5%) deaths from any cause. Two of these deaths were attributed to late-stage oncologic disease, one to acute chest syndrome in a patient with sickle cell disease and one to direct complications of COVID-19 after prolonged respiratory failure (Table 1).
Cardiac testing
Cardiac biomarkers
High-sensitivity troponin T was measured in 27/80 (34%) patients. The median baseline high-sensitivity troponin T was 5 (range 5–28) ng/L and was elevated in 3/27 (7%) patients. The median peak high-sensitivity troponin T was 6 (range 5–103) ng/L and was elevated in 3/27 (11%) patients. N-terminal pro-brain type natriuretic peptide was measured in 10/80 (13%) patients. The median baseline N-terminal pro-brain type natriuretic peptide was 92 (range 4–1351) and was elevated in 3/10 (30%) patients. The median peak N-terminal pro-brain type natriuretic peptide was 127 (range 4–1351) and was elevated in 2/10 (20%) patients. Both high-sensitivity troponin T and N-terminal pro-brain type natriuretic peptide were elevated in 3/5 (60%) of patients with elevated cardiac biomarkers. The decision to admit patients to the ICU or medical–surgical ward was made independent of the results of high-sensitivity troponin T and N-terminal pro-brain type natriuretic peptide testing and all patients admitted to the ICU met other criteria for intensive care admission (Table 2).
Table 2. Results of cardiac testing by location
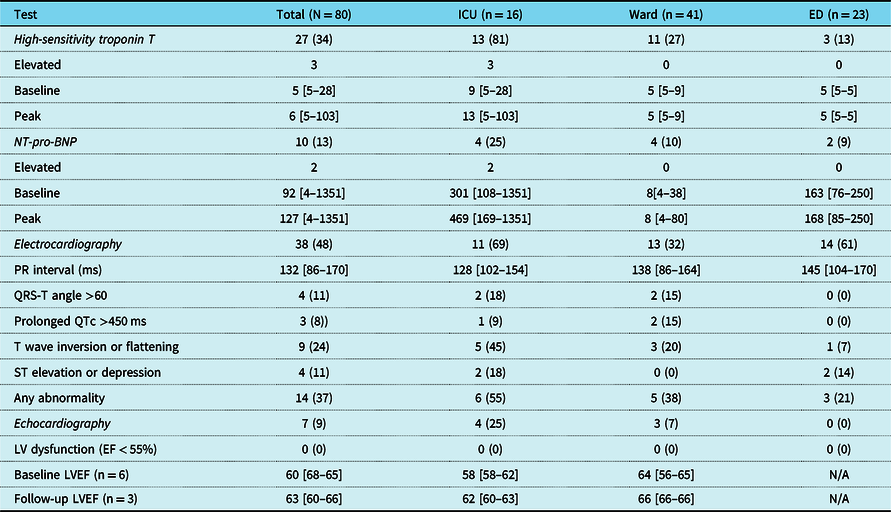
Values represent N (%) for categorical variables and median [range] for continuous variables.
Electrocardiography
At least one ECG was performed in 38/80 (48%) patients. Abnormal findings on baseline ECG were present in 14/38 (37%) patients and included QRS-T angle >60° in 4/38 (11%) patients, QTc >450 ms in 3/38 (8%) patients, T wave flattening or inversion in 14/51 (24%) patients, and ST segment elevations or depressions in 4/38 (18%) patients. There were no patients with documented atrioventricular nodal block.
Echocardiography
At least one echocardiogram was performed during admission for 7/80 (9%) patients, all of which demonstrated normal left ventricular function. Follow-up studies were performed during admission for 2/7 (29%) patients. The median left ventricular ejection fraction on initial echocardiogram was 60% (range 56–65) and on follow-up echocardiogram was 63% (range 60–66). Of the five patients who had echocardiograms performed during admission and survived to discharged, 4/5 had post-discharge echocardiograms performed. Of these, one had a left ventricular ejection fraction of 55% (performed post-chemotherapy) and the rest had left ventricular ejection fractions >55%.
Cardiac biomarkers and outcomes
Elevation in cardiac biomarkers was present in 5/27 (19%) patients for whom high-sensitivity troponin T or N-terminal pro-brain type natriuretic peptide was obtained (Table 3). Underlying comorbidities were present in all five patients with elevated cardiac biomarkers and included diabetes mellitus, Trisomy 21, seizure disorder, Hashimoto’s thyroiditis, spastic quadriplegia, obesity, and obstructive sleep apnoea. All required advanced respiratory support and were admitted to the ICU (Table 4). Severe outcome was experienced by 14/27 (52%) patients for whom cardiac biomarkers were obtained including all 5/5 (100%) patients with elevated cardiac biomarkers. When individual components of severe outcome were analysed for the elevated cardiac biomarker group, 5/5 (100%) received advanced respiratory support, 2/5 (40%) received inotropic or vasoactive support, and 1/5 (20%) died following prolonged respiratory failure. All 5/5 (100%) patients with elevated cardiac biomarkers were admitted to the ICU compared to 8/22 (36%) of those with normal biomarker values. Death occurred in 1/5 (20%) of patients with elevated cardiac biomarkers after a prolonged admission for respiratory failure. Echocardiogram performed on the day of death demonstrated right ventricular hypertension and decreased right ventricular systolic function with preserved left ventricular function.
Table 3. Characteristics and outcomes of patients with and without elevated cardiac biomarkers
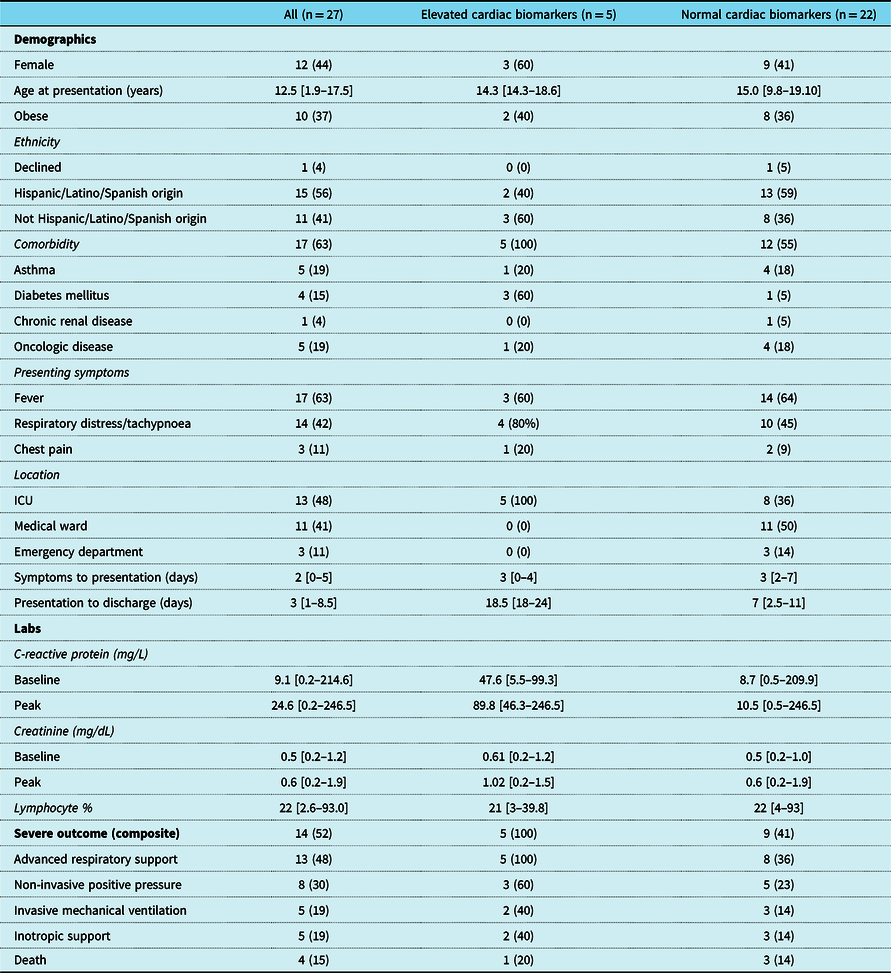
Values represent N (%) for categorical variables and median [range] for continuous variables.
Table 4. Characteristics of patients with elevated cardiac biomarkers
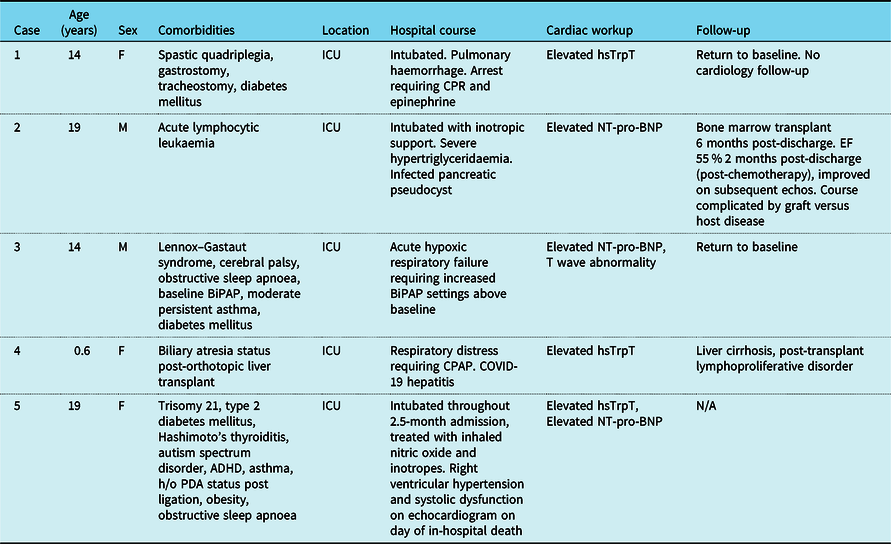
Among those with elevated cardiac biomarkers who survived to discharge, two returned to baseline clinical status. One patient with acute lymphocytic leukaemia went on to undergo bone marrow transplant 6 months post-discharge, subsequently complicated by graft-vs-host disease. One patient with biliary atresia status post-liver transplant had significant hepatitis during admission and was found on biopsy following discharge to have evidence of liver cirrhosis as well as early stages of post-transplant lymphoproliferative disorder.
Discussion
Previously, we described our approach to the cardiac evaluation of children with COVID-19 based on expert opinion. In this institutional protocol, we recommended obtaining at minimum a baseline high-sensitivity troponin T and N-terminal pro-brain type natriuretic peptide in addition to basic inflammatory markers (as per institutional protocol) for these patients. Should any cardiac concerns arise based on history, exam, or other clinical findings, an ECG should be performed, and cardiology consulted, with performance of echocardiography left to the discretion of the consulting cardiologist. Reference Fremed, Lytrivi and Liberman19 Patients with suspected multi-system inflammatory syndrome in children should undergo more complete cardiac workup based on institutional protocol. This protocol was based on expert opinion and was not uniformly implemented at our institution at the time.
We found that among those for whom cardiac biomarkers were measured, these markers were elevated in 19% of patients. Those with elevated cardiac biomarkers were all admitted to the ICU and all experienced severe outcomes including advanced respiratory support. There was one death in the elevated cardiac biomarker group that occurred well after the study period and was attributed to prolonged respiratory failure secondary to COVID-19. We also found electrocardiographic abnormalities in 37% of the total patient population. No patients demonstrated left ventricular dysfunction on echocardiogram, though relatively few underwent echocardiography during that time. Importantly, all patients with elevated cardiac biomarkers had significant comorbidities. Given the incidence of cardiac involvement in these patients and its association with severe outcome even in the absence of multi-system inflammatory syndrome in children, we continue to advocate for the inclusion of cardiac workup as a standard part of the initial evaluation of children and adolescents presenting to the hospital with positive SARS-CoV-2. More extensive workup should be considered based on clinical circumstance and the results of the initial testing.
Adult studies have shown evidence of cardiac involvement in up to 30% of cases Reference Smilowitz, Jethani and Chen20 including potentially 20% of asymptomatic to mild cases, Reference Puntmann, Carerj and Wieters21 as well as an association of cardiac involvement with severe outcomes. Reference Bonow, Fonarow, O’Gara and Yancy3–Reference Guo, Fan and Chen5,Reference Li, Pan and Li22,Reference Clerkin, Fried and Raikhelkar23 Similar studies of children with acute COVID-19 have shown elevated pro-brain type natriuretic peptide but have not focused specifically on cardiac biomarkers. Reference Chao, Derespina and Herold24,Reference Zachariah, Johnson and Halabi25 The association of cardiac involvement and elevated cardiac biomarkers with multi-system inflammatory syndrome in children has been more clearly detailed and cardiac involvement may be present in up to 80% of cases. Reference Feldstein, Rose and Horwitz8 Similar to the adult literature, we found increased rates of respiratory support above baseline and admission to the ICU. Unlike adult findings, however, there was no difference in inotropic support or intubation rates. This is likely due to the relatively low occurrence of these events in children even in severe cases. Death was rare when compared to adult outcomes and we likely had too small of a sample size to determine if those with elevated cardiac biomarkers are at increased mortality risk. Interestingly, unlike previous work Reference Chao, Derespina and Herold24,Reference Zachariah, Johnson and Halabi25 which showed worse outcomes in obese patients, elevation in cardiac biomarkers was not associated with obesity in our study.
This study had several limitations. Early in the pandemic, testing was limited due to shortages of resources, incomplete knowledge on the full extent of disease involvement, and need to limit exposure. For this reason, it is likely that several milder cases were missed. Cardiac testing was also not consistently done early on as the extent of cardiac involvement with this disease was unclear and our institution limited echocardiography to only the most severe cases or those with other signs suggestive of cardiac involvement. Additionally, sicker patients such as those admitted to the ICU may have been more likely to have undergone comprehensive laboratory and imaging workups necessary to determine cardiac status as compared to those admitted to the medical/surgical unit or discharged from the emergency department. It is likely that those patients who were not sick enough to undergo cardiac workup did not have significant cardiac involvement, though larger studies with standardised workup protocols would be necessary to determine if this were true. Our definition of elevated cardiac biomarkers was also reliant on adult reference ranges as no normal values for high-sensitivity troponin T and N-terminal pro-brain type natriuretic peptide have been clearly established for children. Due to our small sample size in elevated cardiac biomarker group, definitive conclusions regarding risk factors for severe outcomes compared to those with normal cardiac biomarkers are difficult to make.
At our institution, cardiac biomarkers were elevated in 19% of children and adolescents with COVID-19 who had these markers measured during the initial peak of the pandemic, though overall rates of testing for cardiac biomarker levels were low. All patients in the elevated cardiac biomarker experienced severe outcomes. Electrocardiographic abnormalities were present in over one-third of cases. Based on these findings, larger studies to determine the true incidence of cardiac involvement in children with COVID-19 in the absence of multi-system inflammatory syndrome in children in the broader population would be useful to guide recommendations for standard workup and management and characterise long-term cardiac sequelae.
Acknowledgements
The authors would like to thank Dr Vidhu Thaker for her assistance with obesity classification.
Financial support
Dr Zucker receives salary support from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number 5UM1AI069470-14 and supplement to the award (JZ), K23AI150378 (JZ) and L30AI133789 (JZ).
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and have been approved by the Columbia University Irving Medical Center Institutional Review Board. Waiver of informed consent was granted by the Institutional Review Board of Columbia University Irving Medical Center in the setting of the ongoing global COVID-19 pandemic.







