Aortopulmonary window is a rare congenital heart defect resulting from malformation of aortopulmonary septum during fetal life. Reference Bertolini, Dalmonte, Bava, Moretti, Cervo and Marasini1 The incidence ranges from 0.05 to 5%. Reference Gowda, Gajjar and Rao2 Up till late 1990s, all aortopulmonary window was surgically closed using various techniques. The results have been excellent though there have been infrequent reports of immediate and late residual defect. Reference Gowda, Gajjar and Rao2 Re-operation in these patients is cumbersome due to excessive adhesions with additional risk of bleeding or vascular trauma. Since late 1990s, small to moderate native and residual aortopulmonary windows are being closed using various devices through percutaneous transcatheter technique with adequate results. Reference Stamato, Benson, Smallhorn and Freedom3–Reference Sadiq, Qureshi, Younas, Arshad and Hyder8 We present, here, the first case of a residual aortopulmonary window using a new Konar-MF occluder device with excellent outcome and conformability.
Case report
A 3-month-old boy presented with heart failure and poor weight gain, and a diagnosis of a large type I aortopulmonary window with severe pulmonary hypertension was made. The defect was surgically corrected using direct ligation technique with smooth post-operative recovery and regression in pulmonary artery pressures. The patient remained well; on follow-up though, he was found to have a small central late residual defect through ligated aortopulmonary window after 9 months. In view of small defect, regressed pulmonary hypertension, and mild left ventricular volume overload, the patient was followed up conservatively.
At 3-year follow-up, the defect was noted to have caused significant left ventricular volume overload with preserved ventricular functions. The defect measured 3–4 mm on echocardiography. The patient was catheterized to delineate the defect, which measured 3.1 mm and 11 mm distal to aortic sinuses in Left Anterior Oblique (LAO)/cranial and Right Anterior Oblique (RAO)/cranial projections (Fig. 1). Haemodynamic assessment showed pulmonary blood flow (Qp):Systemic blood flow (Qs) ratio 2.4:1 with normal pulmonary artery pressures (mean pulmonary artery pressure 18 mmHg). Aortopulmonary window was crossed using a JR4 catheter and a 0.035 exchange length glide wire through aortic end and engaged in distal left pulmonary artery. A 10 mm snare catheter used to snare the wire and railroad was established through right femoral artery. A 7×5 Konar-MF occluder (Lifetech, China) was selected to close the defect. It has a soft woven mesh with a flexible waist primarily designed for perimembranous Ventricular Septal Defect (VSD) closure. It has a double-sided screw for retrograde and antegrade approach and can be delivered through a small delivery sheath. The delivery cable is also slim to avoid untoward damage. Keeping in view, the acute angulation on aortic end entry point, sheath was passed in antegrade fashion. High tensile end was released on aortic side and low tensile end on pulmonary end. The device was released successfully with no residual defect on aortogram (Fig. 2 a, b) or echocardiogram. The flow is seen through the device, which disappeared completely the next day. This relates to the fact that Konar-MF occluder devices up to 8×6 come without a membrane, hence a small delivery sheath (4F, 5F). Sizes 9×7 upwards have a membrane inside the device and would require a relatively large sheath (6F, 7F). The patient was discharged 24 hours after the procedure on aspirin 5 mg/kg daily to be continued for 6 months. Short-term follow-up with repeat transthoracic echocardiogram and cardiac CT at 9 months should demonstrate excellent device profile with no residual leak, no branch pulmonary artery turbulence, and no aortic obstruction (Fig. 2 c, d).

Figure 1. a , b . Ascending aortogram in LAO and RAO projections showing residual shunt across AP window. c . Guide wire snared in LPA. d . Arteriovenous railroad established. e . Delivery sheath passed across AP window and parked in descending aorta. f . Aortic end released. g . Pulmonary end deployed. h. Final position of device after release. AP = Aortopulmonary; LAO= Left Anterior Oblique; LPA = Left Pulmonary Artery; RAO= Right Anterior Oblique.
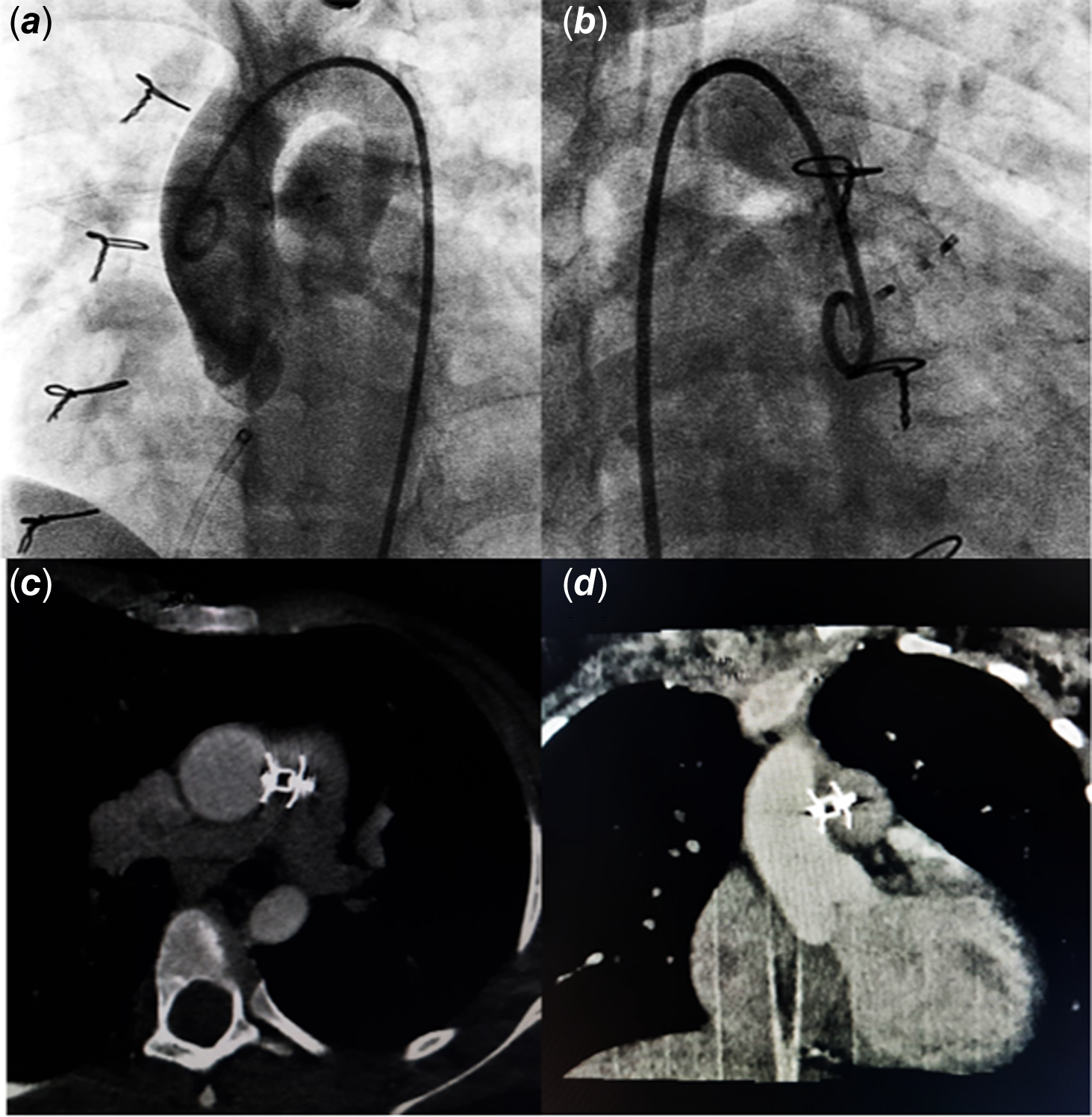
Figure 2. a , b. Post-procedure angiogram. c , d. Follow-up CT angiography.
Discussion
Transcatheter percutaneous occlusion of small to moderate aortopulmonary windows has been done for the last decade with various devices. Reference Stamato, Benson, Smallhorn and Freedom3–Reference Richens and Wilson7 A detailed evaluation of anatomy of the defect is key to decision making firstly whether suitable for device closure or not and then appropriate device selection if deemed suitable for transcatheter closure. Transcatheter closure is considered for isolated defects located away from both semilunar valves and pulmonary artery bifurcation—the type IV or “intermediate” type in the classification scheme recommended by the Society of Thoracic Surgeons Congenital Heart Surgery Database Committee. Double umbrella-shaped devices are considered more suitable as they provide adequate retention skirts on both sides to prevent device dislodgement. None of the devices, however, conform well to the native anatomy resulting in protrusion of either end into aorta or pulmonary artery. Reference Stamato, Benson, Smallhorn and Freedom3–Reference Richens and Wilson7 Furthermore, ventricular or atrial septal occluders carry the risk of protrusion/encroachment of aortic disc onto aortic sinus or cusps.
Konar-MF occluder is soft with a low profile, capable of deployment using small delivery sheath appropriate for small infants through either arterial or venous side and with liberty of choosing high tensile or low tensile disc release on high-pressure end according to anatomical defect configuration. Reference Sadiq, Qureshi, Younas, Arshad and Hyder8 The follow-up CT has shown that it conforms to the anatomy of the defect with very good apposition on either side. A successful closure of a native large aortopulmonary window with pulmonary hypertension in a 12-month-old girl using a large Konar-MF occluder device (12×10) has recently been reported with a good outcome. Reference Abdelrazek Ali, Nour and Rashad9
The nitinol device implanted with a disc in ascending aorta might be the cause of thrombosis with the risk of embolization and ischaemic cerebral events. We used aspirin in anti-platelet dose (5 mg/kg) on the day of procedure to be continued for 6 months as recommended in Indian guidelines after the device closure of an aortopulmonary window. Reference Saxena, Jay and Ravi10
In conclusion, Konar-MF occluder is an excellent new addition to available devices for aortopulmonary window device closure with added adjustability, ease in deployment, and better final profile.
Financial support
None.
Competing interests
None.
Ethical standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and have been approved by the institutional review board of the Children’s Hospital Lahore.





