Children with congenital heart disease (CHD) are at increased risk of a range of psychosocial problems. Therefore, the aim of the current study was to determine the effectiveness of an innovative psychosocial intervention, the Congenital Heart Disease Intervention Program (CHIP)–Family, in improving the psychosocial well-being of children with CHD and their families. The arguments for reducing psychosocial problems in these families are discussed below.
In children with CHD, emotional and behavioural problems may already emerge in infancy.Reference Torowicz, Irving, Hanlon, Fullbright Sumpter and Medoff-Cooper 1 , Reference Solberg, Dale, Holmstrom, Eskedal, Landolt and Vollrath 2 Compared with healthy children, pre-school and school-aged children with CHD have increased levels of internalising and externalising problemsReference Brosig, Mussatto, Kuhn and Tweddell 3 – Reference Karsdorp, Everaerd, Kindt and Mulder 8 and reduced levels of school performance.Reference Martinez-Biarge, Jowett, Cowan and Wusthoff 5 , Reference Hovels-Gurich, Konrad and Skorzenski 7 , Reference McCusker, Armstrong, Mullen, Doherty and Casey 9 Moreover, compared with healthy children, children with CHD more often require remedial teaching or special educationReference Calderon, Bonnet, Pinabiaux, Jambaque and Angeard 10 – Reference Farr, Downing, Riehle-Colarusso and Abarbanell 14 and face increased rates of grade repetition.Reference Bellinger, Newburger, Wypij, Kuban, duPlesssis and Rappaport 4 , Reference Gerstle, Beebe, Drotar, Cassedy and Marino 12 , Reference Bellinger, Wypij and duPlessis 15 Impaired social functioning and social cognition have also been reported.Reference Martinez-Biarge, Jowett, Cowan and Wusthoff 5 , Reference McCusker, Armstrong, Mullen, Doherty and Casey 9 , Reference Cassidy, Ilardi and Bowen 16 Children with CHD participate in fewer social activitiesReference Farr, Downing, Riehle-Colarusso and Abarbanell 14 and are more often perceived to be withdrawn, not accepted by peers, and too dependent on othersReference McCusker, Armstrong, Mullen, Doherty and Casey 9 . In adolescence and adulthood, CHD patients remain at increased risk of psychosocial difficulties.Reference Spijkerboer, Utens, Bogers, Helbing and Verhulst 17 – Reference Holbein, Fogleman and Hommel 22
As to physical activity levels of children with CHD, conflicting results have been reported. Several studies, including two systematic reviews, have reported reduced levels of physical activity in children with severe CHD.Reference McCrindle, Williams and Mital 23 – Reference Duppen, Takken and Hopman 26 However, others have found that physical activity levels of children with different CHD diagnoses do not differ from those of children from the general population.Reference Voss, Duncombe, Dean, de Souza and Harris 27
It is increasingly recognised that parental mental well-being mediates psychosocial outcomes in children with CHDReference Bellinger, Newburger, Wypij, Kuban, duPlesssis and Rappaport 4 , Reference McCusker, Armstrong, Mullen, Doherty and Casey 9 and that family factors are more important predictors of psychosocial outcomes of children with CHD compared to medical factors.Reference Casey, Stewart and McCusker 28 , Reference McCusker, Doherty and Molloy 29 Unfortunately, parents of children with CHD themselves are at increased risk of mental health problems, such as post-traumatic stress disorder, depression, and anxiety.Reference Woolf-King, Anger, Arnold, Weiss and Teitel 30 – Reference McClung, Glidewell and Farr 32
To prevent and decrease these difficulties in children with CHD and their families, a multidisciplinary, psychosocial intervention is needed. Research has demonstrated that families of children with CHD express a need for psychosocial care themselves.Reference Lesch, Specht, Lux, Frey, Utens and Bauer 33 , Reference Levert, Helbing, Dulfer, van Domburg and Utens 34 Until now, the only evidence-based psychosocial intervention in this field was the CHIP-School program.Reference Tesson, Butow, Sholler, Sharpe, Kovacs and Kasparian 35 , Reference McCusker, Doherty and Molloy 36 CHIP-School consisted of a multidisciplinary 1-day group workshop and individual follow-up session for parents of children with CHD who were entering school. The theoretical rationale of CHIP-School was derived from Thompson’s transactional stress and coping model.Reference Thompson, Gustafson, Hamlett and Spock 37 This model states that the effect of an illness on a child’s well-being is mediated by familial coping and appraisal. The developers aimed to strengthen parental mental health and parenting skills, thereby indirectly increasing emotional resilience of children with CHD. CHIP-School yielded positive results with regard to maternal mental health, perceived strain on the family, and school absence of the child. With regard to child emotional and behavioural problems, no significant improvements were found. However, CHIP-School did not contain a specific child module. We reasoned that directly targeting both parents and children would improve the results previously obtained through CHIP-School.
Therefore, we have modified CHIP-School and added a specific child module focused on improving emotional well-being, sports enjoyment, and school functioning. As mothers, fathers, and siblings are also part of this innovate and extended intervention, the intervention is titled “CHIP-Family.” We hypothesised that participating in the CHIP-Family intervention would improve the psychosocial well-being of children with CHD and their parents, family functioning, and parents’ disease-specific knowledge.
Materials and methods
This single-blinded parallel randomised controlled trial was approved by the Medical Ethics Committee of the Erasmus Medical Center and adhered to the ethical guidelines of the Declaration of Helsinki. Before participation, written informed consent was obtained from all patients’ parents or legal guardians. A detailed description of the study protocol has been published previously.Reference van der Mheen, van Beynum and Dulfer 38
Participants
Children and their families were recruited during a 1-year inclusion period (30 September 2016 to 12 September 2017) via the Erasmus Medical Center–Sophia Children’s Hospital, a tertiary referral centre for paediatric cardiology and cardiac surgery in the Netherlands, and nationally via the Dutch Patient Association for Congenital Heart Disease. Families of children who (1) underwent at least one invasive medical procedure for CHD (i.e., cardiac catheterisation and/or open heart surgery) and (2) were attending kindergarten or first or second year of primary school at the time of first assessment and were eligible for participation. Children with known intellectual impairment (intelligence quotient ≤ 70) were excluded, as a sufficient level of intelligence was required to participate in the child intervention program. Moreover, prematurely born children (i.e., gestational age at birth < 37 weeks) with no CHD other than a patent ductus arteriosus were excluded, as families of prematurely born children experience different psychosocial problems.Reference Saigal and Doyle 39 Lastly, sufficient mastery of the Dutch language was required.
Procedure
As to patients of the Erasmus Medical Center, eligibility was assessed by screening patient records of 2- to 8-year-old children who had undergone an invasive cardiac procedure or who had received cardiac follow-up. Subsequently, parents of children who seemed to be eligible received an information letter explaining the purpose and content of the study. As to members of the Dutch Patient Association for Congenital Heart Disease, for privacy reasons, no medical information was available. Therefore, information letters explaining the purpose and content of the study were sent to parents of all 2- to 8-year-old children.
If parents indicated to be interested in participation or did not respond within 2 weeks, eligibility was verified via a phone call. Before giving written informed consent, all families received a verbal explanation of the study and were invited to ask questions. Consequently, an independent researcher randomly assigned participants to the CHIP-Family intervention or care-as-usual control group, which only received medical care (allocation ratio 1:1). The researcher who collected all assessments and performed all analyses was blinded for randomisation outcome. Randomisation was stratified by school year (kindergarten versus primary school) and CHD severity. CHD severity was divided into limited to no residual heart defects versus mild to severe residual heart defects after cardiac intervention (see Table 1). This classification was made based on treatment-related aspects and intensity of cardiac follow-up.Reference Hoffman and Kaplan 40 Randomisation block size was fixed at four per stratification category. Due to logistical reasons in the starting phase of the project, the first four families who consented to participate were allocated to the CHIP-Family group without randomisation. We limited the period between baseline assessment and the intervention to 2 weeks. Moreover, parents had to be notified earlier that they had to make practical arrangements to be able to participate in the CHIP-Family workshop for an entire day. For these two important logistic reasons, parents of patients were informed of randomisation outcome prior to the baseline assessment. The follow-up assessment took place 6 months after baseline. The participation flow chart is shown in Fig 1.
Table 1. Stratification factor “Congenital Heart Disease Intervention Program (CHD) severity.”
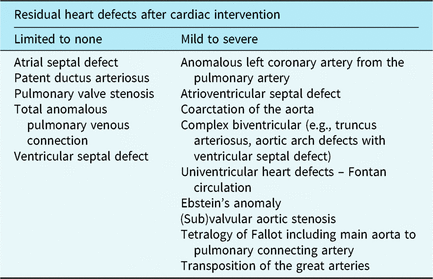
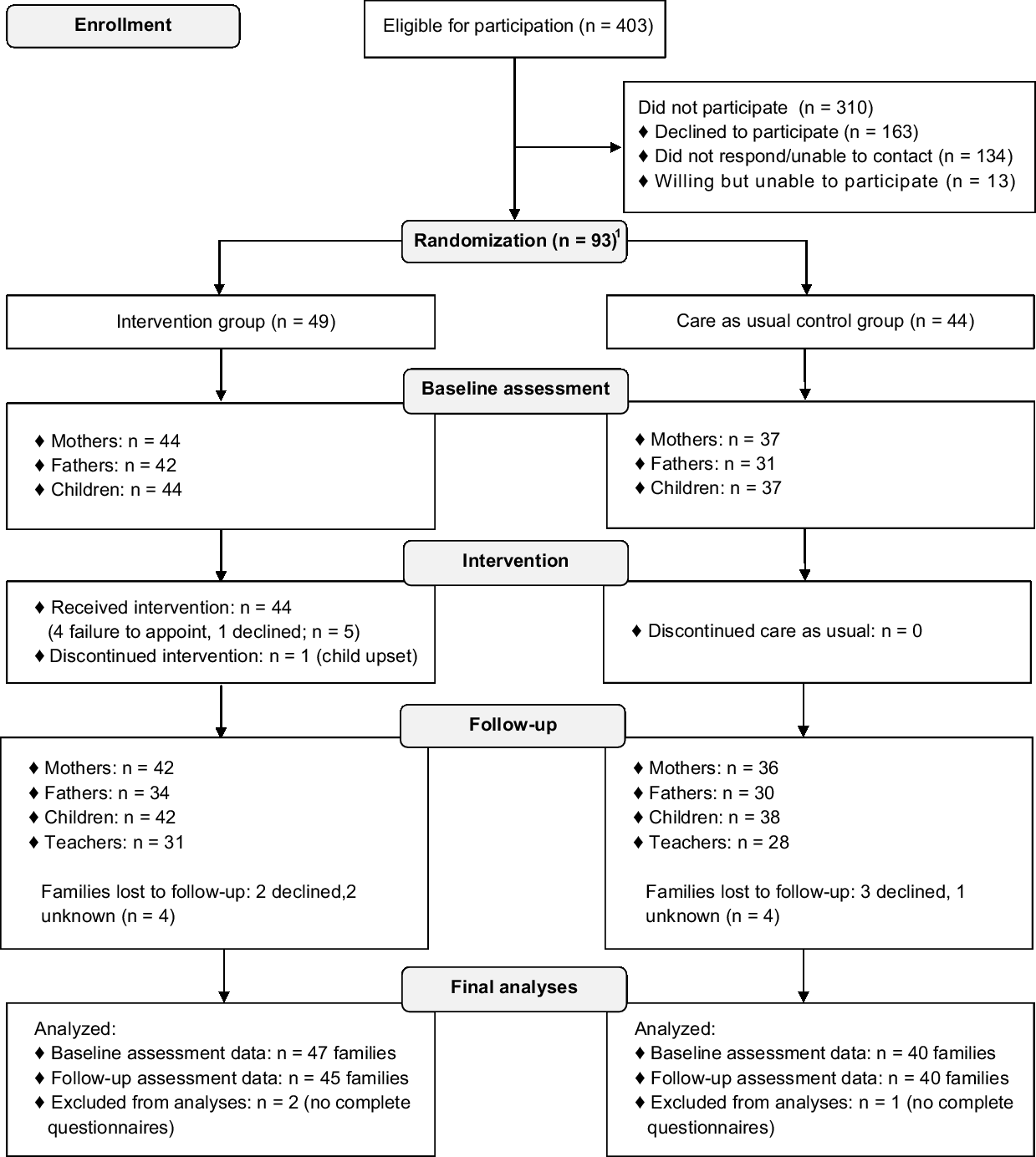
Figure 1. Participation flow chart.
Intervention
The CHIP-Family is an adaptation and extension of the CHIP-School intervention.Reference McCusker, Doherty and Molloy 36 CHIP-Family consisted of a 6-hour group workshop (three to five families per workshop) for parents and children and an individual 1-hour follow-up session per parent couple. The developers of the original CHIP protocol conducted a 1-day training for four senior licensed clinical and health psychologists and five junior psychologists to deliver the parent module of the CHIP-Family intervention. A senior psychologist trained the junior psychologists to provide the child module. During the parent workshops and child workshops (one student per workshop), protocol adherence was assessed through a standardised form by psychology master’s students. For privacy reasons and due to the group format of the CHIP-Family workshops, it was not possible to videotape or audiotape the workshops. With the consent of parents, protocol adherence of the individual follow-up sessions was assessed through audiotapes by psychology master’s students.
The 1-day parent workshop consisted of problem prevention therapy, psychoeducation, general parenting skills, skills specific to parenting a child with CHD (provided by two senior clinical psychologists for 4 hours), and medical issues (provided by a paediatric cardiologist supported by a senior clinical psychologist for 1 hour). The 1-hour lunch break gave families more opportunity to share their experiences.
As background information, parents received all slides that were presented in the workshop, the CHIP manualReference Utens, Dulfer, Legerstee, Van Beynum, Meentken and Van der Mheen 41 containing all topics covered in the workshop, several information leaflets, and a home assignment on problem prevention therapy. Approximately 4 weeks after the workshop, each parent couple received an individual follow-up session provided by a senior psychologist who was present at the parent workshop and a psychologist who was present at the child workshop. The follow-up session focused on questions or worries of individual families, future coping strategies, and the problem prevention home assignment.
Whereas the CHIP-School intervention consisted of a parent module, the CHIP-Family module also comprised a specific child module. The child module consisted of a workshop that was held concurrently with the parent workshop. The child workshop consisted of cognitive behavioural exercises based on the evidence-based Fun FRIENDS protocolReference Utens 42 , Reference Barrett 43 and focused on strengthening self-esteem, regulating emotions, relaxation, problem-solving skills, and positive thinking (provided by two junior psychologists who were supervised by two senior clinical psychologists for 4 hours). The children also did sport exercises based on a standardised exercise programReference Dulfer, Duppen and Kuipers 44 specifically developed for children with CHD and their siblings (provided by a physiotherapist and assistant for 1 hour). Each child was allowed to bring a 4- to 10-year-old sibling or friend to normalise participation and to stimulate practice at home.
Thus, though predicated on a similar conceptual model, CHIP-Family differed from CHIP-School by having parallel modules/workshops for the whole family (children, siblings, and parents). In addition, CHIP-School included a bicycle exercise stress test. This was essentially a behavioural experiment to highlight to parents (in vivo and in the presence of a cardiologist) that vigorous exercise was safe with non-concerning electrocardiogram rhythms evident throughout. Unfortunately, it was not possible to include this in the current CHIP-Family intervention for logistic reasons.
Instruments
All questionnaires were completed by both parents at baseline and 6-month follow-up, unless otherwise specified. Teachers completed only the 6-month follow-up questionnaires, because some teachers did not know the child sufficiently at baseline to fill out the questionnaires. If a child had multiple teachers, questionnaires were completed by the teacher who knew the child the best or spent the most time with the child. Children completed two sports-related questions at baseline and 6-month follow-up. Validated Dutch versions of internationally well-known questionnaires with adequate psychometric properties were used. All questionnaires were completed through a secure online system. Demographic variables were assessed through the Rotterdam Quality of Life interview.Reference Utens and Dulfer 45
Child outcomes
Child emotional and behavioural problems were the primary child outcome and were assessed through the Child Behavior ChecklistReference Achenbach and Rescorla 46 1½-5 (100 items; for 4- and 5-year-olds) or Child Behavior Checklist 6-18 (120 items; for 6- to 8-year-olds).
Problem behaviour at school was assessed through the Teacher’s Report Form-CReference Achenbach and Rescorla 46 1½-5 (100 items; for 4- and 5-year-olds) or the Teacher’s Report Form 6-18Reference Verhulst and van der Ende 47 (120 items; for 6- to 8-year-olds) which was completed by teachers.
Executive functioning was assessed through the Behavior Rating Inventory of Executive FunctioningReference Smidts and Huizinga 48 (63 items; for 6- to 8-year-olds) or Behavior Rating Inventory of Executive Functioning–Preschool versionReference Van der Heijden, Suurland, De Sonneville and Swaab 49 (63 items; for 4- and 5-year-olds) which was completed by parents and teachers.
Children’s health-related quality of life was assessed through the Child Health Questionnaire–Parent Form-50Reference Langraf, Abetz and Ware 50 (50 items).
School absence was assessed through the Rotterdam Quality of Life interview.Reference Utens and Dulfer 45
Children’s enjoyment of physical activity was assessed through an adjusted version of the Groningen Enjoyment QuestionnaireReference Stevens, Moget, de Greef, Lemmink and Rispens 51 , Reference Dulfer, Duppen and Blom 52 (10 items) which was completed by parents and teachers. The sentencing of the Groningen Enjoyment Questionnaire items was adjusted to enable parents and teachers to fill out the questionnaire (e.g., “This child likes being physically active” instead of “I like being physically active”). In addition to the Groningen Enjoyment Questionnaire, children themselves were asked to answer two questions indicating their enjoyment of physical activity and how often per week they engage in physical activity.
Parental and family outcomes
Parental mental health was the primary parental outcome and was assessed through the Symptom Checklist-90-RevisedReference Arrindell and Ettema 53 (90 items), which measures symptom severity of mental health problems.
Excessiveness and uncontrollability of parental worry were assessed through the Penn State Worry QuestionnaireReference Meyer, Miller, Metzger and Borkovec 54 , Reference Van der Heiden, Muris, Bos and Van der Molen 55 (16 items).
Parenting stress was assessed through the short version of the Nijmeegse Ouderlijke Stress IndexReference De Brock, Vermulst, Gerris and Abidin 56 (25 items) and the Distress Thermometer-PReference Haverman, van Oers and Limperg 57 (42 items).
Parents’ health-related quality of life was assessed through the Short-form (36) Health SurveyReference Aaronson, Muller and Cohen 58 (36 items).
Family functioning was assessed through the general functioning subscale of the Family Assessment DeviceReference Epstein, Baldwin and Bishop 59 (12 items).
Parents’ knowledge about CHD (10 items) were assessed through the Rotterdam Knowledge Questionnaire for Congenital Heart Disease.Reference Dulfer, Duppen and Blom 52 , Reference Utens and Dulfer 60
Program satisfaction was assessed through a social validity questionnaire that parents completed 2 weeks after CHIP-Family and at 6-month follow-up.
Statistical analyses
Differences in baseline participant characteristics between the CHIP-Family and the care-as-usual groups were examined using t-tests, chi-square tests, and Fisher’s exact tests, where appropriate.
To determine the effectiveness of the CHIP-Family intervention, we compared the differences in change in parent- and child-reported outcomes over time between the CHIP-Family and care-as-usual group using generalised estimating equations.Reference Zeger, Liang and Albert 61 The generalised estimating equation analysis accounts for the dependency between repeated observations and accommodates missing values.Reference Twisk 62 The analyses were performed on an intention-to-treat basis. Patients were included in an analysis if an outcome was available at one or both assessment moments. For each outcome, we conducted separate generalised estimating equation analyses. The interaction between time and group (i.e., CHIP-Family versus care as usual) was examined as the test of effectiveness of CHIP-Family. We selected a normal distribution and an identity link function for the majority of outcomes. The Symptom Checklist-90-Revised total score, Distress Thermometer-P total score, Short Form-36 physical component score, pleasure in sports reported by children, and sports participation per week were not normally distributed. Therefore, for these outcomes a gamma distribution with log link function was used.
Teacher-reported outcomes were only assessed at follow-up. We used t-tests to compare the difference in teacher-reported continuous outcomes between the CHIP-Family and care-as-usual group. Because the majority of children had not been absent from school the past month, school absence was dichotomised into 0 days absent and ≥1 day absent and presented as percentage. To compare the difference in school absence between the CHIP-Family and care-as-usual group, chi-square tests were applied.
For the primary outcome variables (Child Behavior Checklist and Symptom Checklist-90-R), the significance level was set at α = 0.05. To adjust for multiple testing within the secondary outcome variables of the child domain and the parental and family domain, we used a Bonferroni correction. In both domains, 17 tests were conducted. Therefore, results with p < 0.003 were considered statistically significant. All statistical analyses were performed using SPSS version 24.Reference Corp 63 Sample size calculations can be found in a previous publication of the study design.Reference van der Mheen, van Beynum and Dulfer 38
Results
Participant characteristics
In total, 93 children were randomised into either the CHIP-Family (n = 49) or the care-as-usual group (n = 44). Non-participants’ cardiac diagnosis and gender were only available for patients of the Erasmus MC–Sophia Children’s Hospital (86.6% of eligible patients) and not for patients contacted via the Dutch Patient Association for Congenital Heart Disease (13.4% of eligible patients). Participants and non-participants from the Erasmus MC–Sophia Children’s Hospital did not differ as to CHD severity (p = 0.06) and gender (p = 0.12). Other demographic data were not available.
Parents of three children did not complete any questionnaires after randomisation. Therefore, the final sample consisted of 90 children (n = 47 CHIP-Family, n = 43 care as usual). The CHIP-Family and care-as-usual group did not differ from each other in terms of age, gender, CHD type, school year, recruitment centre, comorbid physical illness, family composition, or social economic status (see Table 2), which indicates that randomisation was successful. Four children were referred for further psychological care after participating in the CHIP-Family intervention (n = 44) and received this care after completion of the follow-up assessment. Problems such as anxiety and emotion regulation issues were noted in these children during the group workshop and/or individual follow-up session.
Table 2. Baseline participant characteristics.
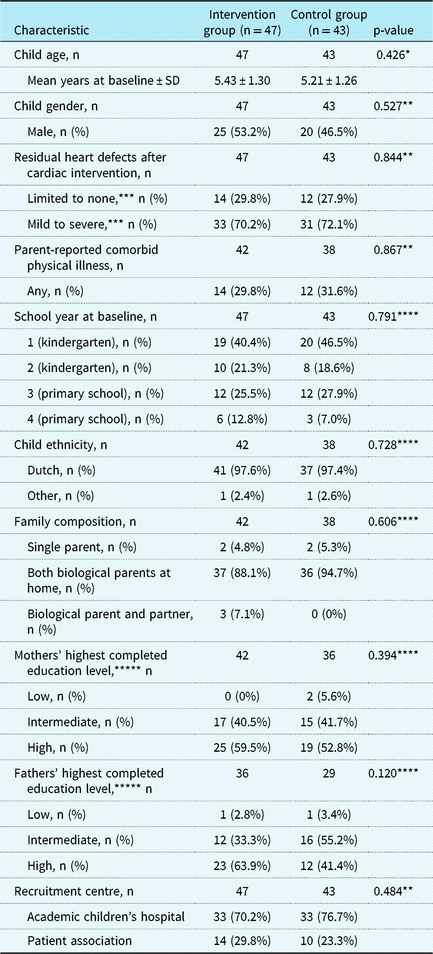
* t-test
** Chi-square test
*** Limited to none: atrial septal defect, patent ductus arteriosus, pulmonary valve stenosis, total anomalous pulmonary venous connection, ventricular septal defect. Mild, moderate, severe: anomalous left coronary artery from the pulmonary artery, atrioventricular septal defect, coarctation of the aorta, complex biventricular (e.g., truncus arteriosus, aortic arch defects with ventricular septal defect), univentricular heart defects – Fontan circulation, Ebstein’s anomaly (sub)valvular aortic stenosis, Tetralogy of Fallot (TOF) including main aorta to pulmonary connecting artery, transposition of the great arteries
**** Fisher’s exact test
***** Low: primary education, lower vocational education, lower or middle general secondary education; intermediate: middle vocational education, higher secondary education, pre-university education; high: higher vocational education, university
Protocol adherence
Workshops
Five families randomised into the CHIP-Family group did not participate in the CHIP-Family intervention. Of these five families, one family declined to participate in CHIP-Family and four families were unable to attend the intervention due to practical reasons. In total, 44 families participated in the CHIP-Family workshops. One family discontinued the intervention after approximately 1 hour, because the child was upset.
Of 34 (77.3%) families, both parents participated in the workshop. Of the remaining 10 (22.7%) families, only the mother participated in the workshop. Twenty-eight (63.7%) children participated in the workshop with a sibling, 6 (13.6%) children participated with a friend, and 10 (22.7%) children participated without a sibling or friend. In nine parent workshops and seven child workshops, all the protocol topics were discussed. In the remaining two parent workshops and four child workshops, 96% of the protocol topics was discussed. This indicates excellent protocol adherence.
Follow-up sessions
Of all families, at least one parent attended the follow-up session. Four psychology master’s students rated protocol adherence of a randomly selected 50% of the follow-up sessions. On average, 87% of the protocol topics was discussed in the randomly selected follow-up sessions.
Outcomes
Child, parental, and family outcomes are summarised in Tables 3 and 4. No statistically significant differences between the CHIP-Family and care-as-usual group were found in both the child outcomes (reported by children, mothers, fathers, and teachers) and the parental and family outcomes (reported by mothers and fathers).
Table 3. Child outcomes.
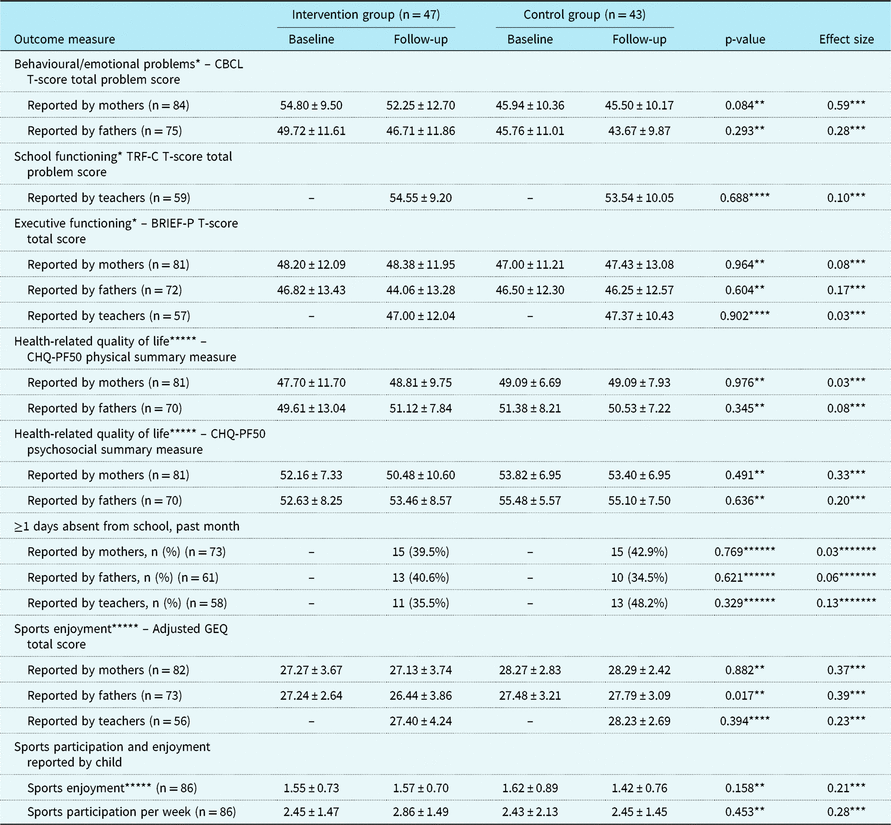
BRIEF-P = Behavior Rating Inventory of Executive Functioning–Preschool version; CBCL = Child Behavior Checklist; CHIP = Congenital Heart Disease Intervention Program; CHQ-PF50 = Child Health Questionnaire–Parent Form-50; GEE = generalised estimating equation; GEQ = Groningen Enjoyment Questionnaire; TRF-C = Teacher’s Report Form-C
Values are mean ± SD, unless otherwise specified
* A higher score indicates more problems or poorer functioning
** GEE analysis. p-Value indicates the level of significance of the interaction between condition and follow-up time
*** Cohen’s d = (MCHIP_T2 – MCAU_T2)/SDpooled. SDpooled = √((SDCHIP_T2 2 + SDCAU_T2 2)/2)
**** T-test
***** A higher score indicates less problems or better functioning
****** Chi-square test
******* Phi coefficient
Table 4. Parental and family outcomes.
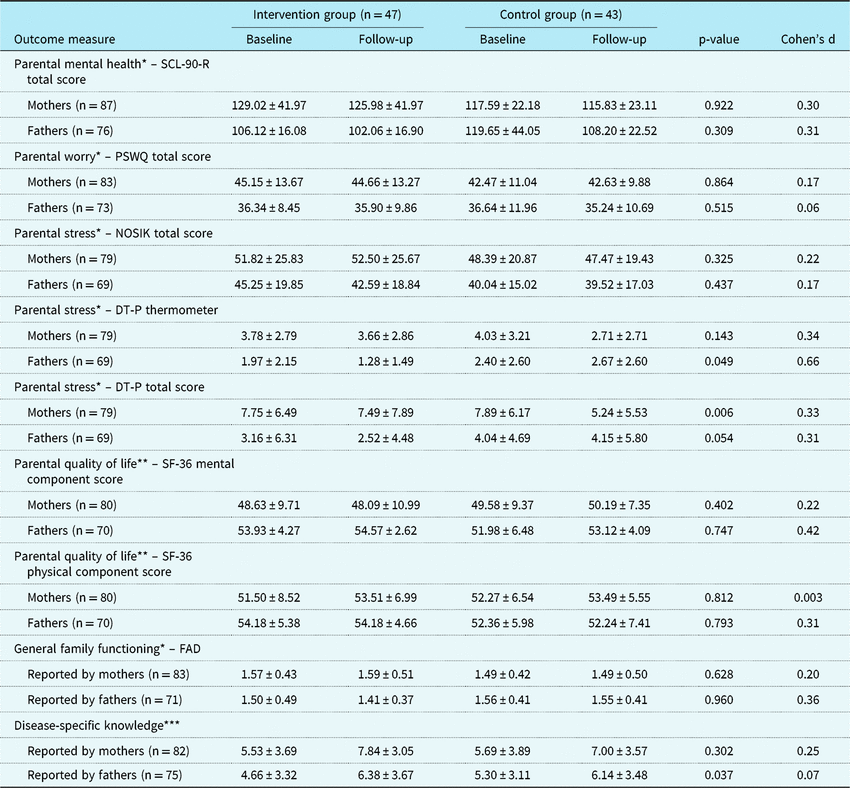
DT-P = Distress Thermometer-P; FAD = Family Assessment Device; NOSIK = Nijmeegse Ouderlijke Stress Index; PSWQ = Penn State Worry Questionnaire; SCL-90-R = Symptom Checklist-90-Revised; SF-36=Short-form (36) Health Survey
Values are mean ± SD. p-Values indicate the level of significance of the interaction between condition and follow-up time
* A higher score indicates more problems or poorer functioning
** A higher score indicates less problems or better functioning
*** A higher score indicates more disease-specific knowledge (maximum score = 14)
Despite successful randomisation, the baseline difference in the Child Behavior Checklist total scores reported by mothers was statistically significant (p = 0.001), such that mothers in the CHIP-Family group reported more child emotional and behavioural problems at baseline than mothers in the care-as-usual group. No other baseline differences in outcomes were found. For both the CHIP-Family and the care-as-usual group, the Child Behavior Checklist total scores reported by both mothers and fathers significantly decreased from baseline to follow-up (p = 0.001 and p < 0.001).
Program acceptability
Thirty-one (70.5%) mothers and 26 (76.5%) fathers who participated in CHIP-Family rated program acceptability at 6-month follow-up. On a scale of 0–10, mean overall usefulness rating was 7.5 (SD = 1.6) and 7.8 (SD = 1.5), respectively, for the parent and child workshop. Mean satisfaction rating was 7.7 (SD = 1.2) and 8.1 (SD = 1.3) for the parent and child workshop, respectively. Mean rating of the usefulness of the individual follow-up session was 7.4 (SD = 1.5). Mean rating of the likeliness that parents would recommend CHIP-Family to other families of children with CHD was 7.7 (SD = 1.5). Parents were asked to rate the components of CHIP-Family that they found most useful (see Fig 2). Most parents perceived the psychosocial and medical explanation of the paediatric cardiologist (72.7%), meeting other families of children with CHD (61.8%), the child workshop (50.9%), and receiving skills tailored to parenting children with CHD (43.6%) as the most useful elements of the intervention. At follow-up, a substantial percentage of parents (47.7%) reported using the techniques learnt in CHIP-Family sometimes (monthly).
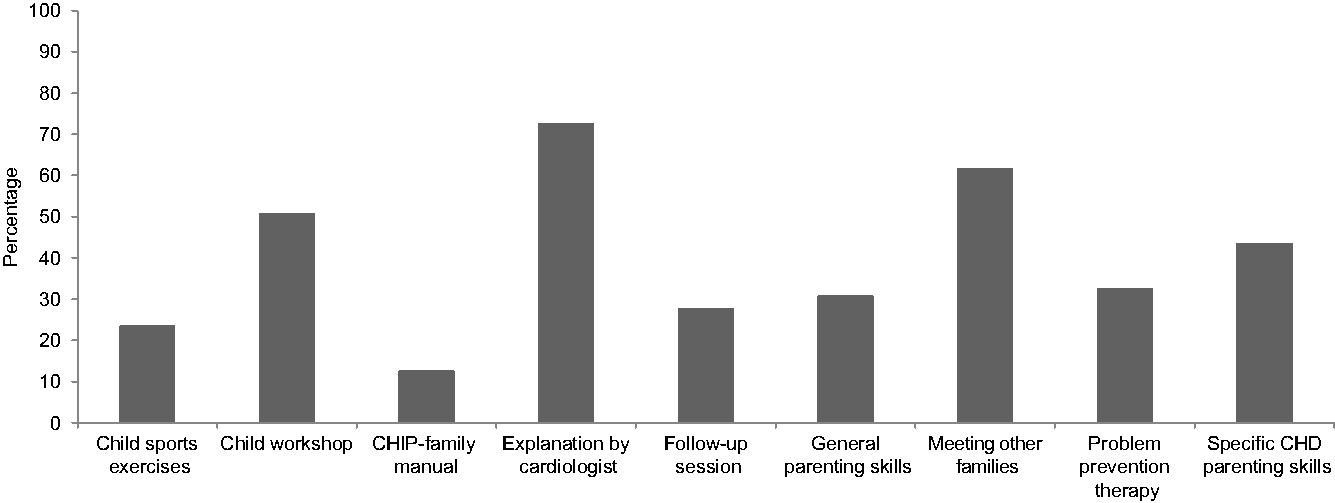
Figure 2. Parents’ ratings of most useful components of the Congenital Heart Disease Intervention Program (CHIP)-Family intervention (multiple answers possible).
Parents of children with less severe CHD (i.e., limited to no residual heart defects after cardiac intervention; see Table 1) rated usefulness of the child workshop more favourably (M = 8.4, SD = 1.6) compared to parents of children with more severe CHD (i.e., mild to severe residual heart defects after cardiac intervention; see Table 1; M = 7.4, SD = 1.4), t(53) = 2.23, p = 0.03. Parents of children with less severe CHD also rated satisfaction with the child workshop more favourably (M = 8.6, SD = 1.3) compared to parents of children with more severe CHD (M = 7.8, SD = 1.2), t(53) = 2.47, p = 0.02.
Discussion
The aim of the current randomised controlled trial was to examine the effect of the multidisciplinary, psychosocial CHIP-Family intervention on psychosocial well-being of young children with CHD and their families. Parents evaluated usefulness of and satisfaction with CHIP-Family positively. Moreover, through CHIP-Family, the involved mental healthcare professionals were able to identify four children who had psychosocial issues that required additional psychological care. These psychosocial issues might otherwise have remained unnoticed and untreated. However, our findings indicate that, compared with care as usual, participation in CHIP-Family did not significantly improve the psychosocial well-being of children with CHD and their families at 6-month follow-up. This is in contrast with the results of the previously examined CHIP-School intervention, which yielded more positive results at 10-month follow-up.Reference McCusker, Doherty and Molloy 36
The culture of replicability research in psychological interventions in general is poor and publication bias often amplifies the problem.Reference Hughes 64 However, replication studies are important not only to confirm or question the impact of an intervention but rather to yield important information to further refine interventions and evaluation protocols. Thus, Stehl and colleaguesReference Stehl, Kazak and Alderfer 65 found that a similar family-focused intervention for parents of children with cancer, whilst successful later in the illness trajectory, yielded less impressive outcomes when delivered earlier in the illness cycle – despite the promising theoretical reasons why it might be even more effective. Law and colleagues’Reference Law, Fisher, Fales, Noel and Eccleston 66 meta-analysis highlighted generally small to moderate effect sizes, and parental functioning in family-focused interventions ultimately aimed at improving outcomes for the child. Again, important lessons in intervention focus and measurements used were discussed.
Following the results of the present study, we were interested in considering differences between CHIP-Family and the previous CHIP-School on which it was based, and what these might tell us about future interventions and evaluations. Two classes of differences may be important:
The intervention – Some key differences may have been important. Firstly, as noted above, CHIP-School comprised a behavioural experiment – the bicycle exercise test – to directly challenge assumptions about fragility and poor exercise capacity. For logistical reasons, ours did not. CHIP-School authors noted that parents had rated this component as the most helpful (personal communication). Secondly, we incorporated parallel workshops for children and siblings. Whilst theoretically we expected this to enhance impact, having their children attend the same day may in fact have diluted the importance of, and engagement with, the primacy of the parent focus of the intervention. It should be noted, however, that parents did rate the child workshop positively. Finally, we had smaller groups of parents (three to five) compared to CHIP-School. Again, we expected this to enhance impact, but such may also have moderated the social facilitation and support impact of the groups.
The sample – In CHIP-Family, we had lower rates of uptake (24%) compared to CHIP-School (60%). Although our responders seemed similar to non-responders (see above) on CHD severity measures, our samples may have differed on other important psychosocial ways which our studies are not able to compare but which relate to the issue of targeting such interventions. It is difficult to draw clear conclusions here, but this merits consideration in future research. CHIP-School targeted families just before the child made the transition to school, whereas CHIP-Family included families of children who had already started school. DrotarReference Drotar 67 makes the case for timing of early interventions at the cusp of developmental transitions and this may be important.
As CHIP-Family was designed as a preventive intervention, patients and their families were not selected for participation in the randomised controlled trial based on their level of psychosocial difficulties. Considering the baseline scores, participants seemed to be functioning relatively well. That is, compared with the general population, all mean scores fell within the normal range. One might expect that baseline scores would have been higher if we would have included only children with severe or complex CHD. However, a meta-analysisReference Karsdorp, Everaerd, Kindt and Mulder 8 has shown that disease severity in children with CHD is not related to internalising, externalising, and overall emotional and behavioural problems. Also, the majority of parents were highly educated. Significant improvements might have been found if we provided CHIP-Family specifically to patients and families who suffered from clinically significant psychosocial difficulties.
Moreover, besides issues related to early childhood, several topics were discussed in the CHIP-Family intervention, concerning future issues related to adolescence and young adulthood, such as alcohol use, smoking, sexuality, insurances, and career possibilities. This was done to provide parents an overall future perspective and also to encourage parents to ask for advice or help from the medical staff. Furthermore, these were topics often addressed by parents during the intervention. Whether this kind of psychoeducation has positive effects when these children reach adolescence could not be assessed within the shorter 6-month follow-up period.
Remarkably, although no differences in participant characteristics were found between the CHIP-Family and the care-as-usual group, a baseline difference was found in child emotional and behavioural problems reported by mothers. Mothers in the CHIP-Family group reported more child emotional and behavioural problems than mothers in the care-as-usual group. This might be explained by the fact that participants were aware of randomisation outcome prior to baseline assessment. Due to logistical reasons, this could not be arranged otherwise. Parents might have psychologically prepared for the CHIP-Family intervention by reflecting on questions they wanted to discuss, which may have increased their awareness of problems and, consequently, increased their problem reports.
Moreover, we found that both mother reports and father reports of the Child Behavior Checklist questionnaire in the care-as-usual and CHIP-Family group improved similarly over time. This might be explained by the “question–behaviour effect”. That is, behaviour of participants can be affected by merely filling out questionnaires,Reference Wilding, Conner and Sandberg 68 which was done by parents of children in both the care-as-usual and the CHIP-Family group. Furthermore, the information letters sent to potential participants contained information on common psychosocial issues in young children with CHD. Reading this information may have had a normalising effect. Also, perhaps the feeling of receiving more attention from the hospital staff by participating in the study may have contributed to positive outcomes for both groups.
Interestingly, we found that parents of children with less severe CHD rated the usefulness of and satisfaction with the child workshop more favourably than parents of children with more severe CHD. This could be attributed to the fact that children with less severe CHD make less outpatient clinic visits compared to children with more severe CHD. Parents of children who make less clinic visits might appreciate the attention of healthcare professionals more compared to parents of children who are accustomed to more frequent visits to clinic. Alternatively, parents of children with more severe CHD might prefer a different child intervention program than parents of children with less severe CHD.
Strengths and limitations
This study has several strengths. Worldwide CHIP-Family is the first psychosocial intervention for children with CHD, which is comprised of both a specific child and parent module. Protocol adherence was strong. Moreover, fathers are underrepresented in paediatric research and, when fathers are included, mothers’ and fathers’ reports often are not analysed separately.Reference Phares, Lopez, Fields, Kamboukos and Duhig 69 , Reference Parent, Forehand, Pomerantz, Peisch and Seehuus 70 Both mothers and fathers participated in CHIP-Family and their outcome reports were analysed separately.
A number of limitations should also be considered. Firstly, as mentioned above, informing participants of randomisation outcome prior to the baseline assessment may have influenced the results. Secondly, due to the nature of the intervention, it was not possible to blind participants for group status. Thirdly, the differences in outcome scores in favour of the CHIP-Family group might have been statistically significant if the sample size would have been larger. Finally, perhaps we would have found larger effects if we had used more disease-specific questionnaires related to family functioning and worry. However, at the start of this study, these tools were not available in Dutch.
Conclusion
In summary, CHIP-Family was evaluated positively by participants and seems to meet parents’ and patients’ needs. However, the intervention did not significantly improve the psychosocial well-being of young children with CHD and their families at 6-month follow-up. As CHIP-Family did not meet its expectations, future research should focus on which patients and families will benefit most from a psychosocial intervention. Future research should also examine whether intervention programs should be adjusted according to CHD severity. Moreover, alternative formats in which psychosocial interventions may be provided could be considered, such as easily accessible online psychoeducation or group videoconferences. Also, psychosocial topics could be integrated into shared medical appointments,Reference Kirsh, Aron and Johnson 71 which show promising results.
Acknowledgements
We are grateful to all families that participated in this trial and thank the Dutch Patient Association for Congenital Heart Disease for their advice and their help in facilitating the conduct of this trial. We also thank all psychologists, paediatric cardiologists, physiotherapists, and master’s students for their efforts in providing the CHIP-Family intervention.
Financial Support
This work was supported by Fonds NutsOhra, Amsterdam, the Netherlands (grant number 101.083). The funding source had no role in the design of the study, its execution, analysis, interpretation of the data, or decision to submit results.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by Medical Ethics Committee of the Erasmus Medical Center. Informed consent was obtained from all patients in accordance with the university hospital policies.








