Introduction
According to the latest report of the Alzheimer Society of Canada (2016), more than half a million Canadians are currently living with dementia, a number expected to double by 2031. Each year, one in three older adults (65 years of age and older) dies with dementia (Canadian Academy of Health Sciences, 2019). This is the result of a host of factors, including population aging, the lack of truly effective ways to prevent or cure neurodegenerative disorders, and medical advances that have reduced other causes of death, such as cancer and cardiovascular diseases (Tejada-Vera, Reference Tejada-Vera2013). Providing quality care to patients dying with dementia is therefore increasingly important. Most people want to die at home (Gomes, Calanzani, Gysels, Hall, & Higginson, Reference Gomes, Calanzani, Gysels, Hall and Higginson2013). However, because care needs of people with dementia are often complex, families are seldom able to keep their loved ones at home up until they die. In many countries, including Canada, the vast majority of people with dementia die in a long-term care (LTC) facility (Reyniers et al., Reference Reyniers, Deliens, Pasman, Morin, Addington-Hall and Frova2015). In Canada, more than 85 per cent of LTC residents have cognitive impairment (Estabrooks et al., Reference Estabrooks, Straus, Flood, Keefe, Armstrong and Donner2020).
Knowledge about the quality of end-of-life care for people with dementia has progressed significantly over the past few decades (Mitchell, Reference Mitchell2020). Studies have shown that the care provided is often less than optimal, because of inadequate symptom management and the use of burdensome interventions with limited clinical benefit (e.g., Hendriks, Smalbrugge, Hertogh, & van der Steen, Reference Hendriks, Smalbrugge, Hertogh and van der Steen2014; Lee et al., Reference Lee, Sussman, Kaasalainen, Durepos, McCleary and Wickson-Griffiths2020; Sampson et al., Reference Sampson, Candy, Davis, Gola, Harrington and King2018; Vandervoort et al., Reference Vandervoort, Van den Block, van der Steen, Volicer, Vander Stichele and Houttekier2013). Current knowledge, however, originates mostly from European countries and the United States, with no data routinely collected in Canada on the quality of care provided to LTC residents dying with dementia (Estabrooks et al., Reference Estabrooks, Straus, Flood, Keefe, Armstrong and Donner2020). Current knowledge gaps hinder our ability to ensure end-of-life quality for a growing and vulnerable population, as the COVID-19 crisis has sadly revealed. Between March 1, 2020, and February 15, 2021, 69 per cent of all reported COVID-19 deaths in Canada occurred in LTC and retirement homes (Canadian Institute for Health Information, 2021), a proportion higher than in other countries (Canadian Institute for Health Information, 2020). Forty-five per cent of COVID-19 deaths in Canada occurred in Quebec (Government of Canada, 2021), the province with the highest percentage of older adults living in LTC homes (Canadian Institute for Health Information, 2020). The COVID-19 pandemic has also had a unique impact on residents living with dementia. This population has been shown to be at greater risk of contracting COVID-19, and of experiencing worse clinical outcomes once infected, than people without dementia (Wang, Davis, Gurney, & Xu, Reference Wang, Davis, Gurney and Xu2021). Residents with dementia have also been particularly affected by measures taken to control the spread of the virus, such as the ban on visits from close relatives (Numbers & Brodaty, Reference Numbers and Brodaty2021).
In response to the need for high-quality and comprehensive data on the last phase of life of LTC residents with dementia, the Quebec Observatory on End-of-Life Care for People with Dementia was created. The Observatory is a research infrastructure set up with the primary objective of supporting the collection of data needed to better understand, and subsequently enhance, the quality of care provided to LTC residents dying with dementia in the province of Quebec. This article reports on the main steps involved in setting up the Observatory, as well as a pilot study conducted within this research infrastructure. It describes the pilot data, methodological changes that were made along the way, feedback from participating facilities, and future developments of the Observatory.
Setting up the Observatory
After obtaining funding from various sources for implementing the Observatory, Drs. Bravo and Arcand reached out to researchers from other research centres on aging located in the province of Quebec who had an interest in end-of-life care for residents with dementia and complementary expertise in related areas, such as pain assessment, communication challenges, and medication appropriateness. Those interested in joining the principal investigators to work together on issues related to dementia end-of-life care became part of the End-of-Life Care in Dementia Research Group.
Achieving the Observatory’s primary objective of supporting data collection on the care delivered to residents dying with dementia required developing a close partnership with LTC facilities, and implementing standardized data collection at these sites on the provision of dementia end-of-life care. From April 2015 to March 2016, assisted by a research coordinator with extensive knowledge of Quebec’s LTC sector, the Research Group identified LTC facilities likely to cater to residents with dementia until their eventual death, established recruitment strategies and methods of data acquisition, selected variables of primary interest and determined how best to measure them, developed electronic tools to allow for less costly data collection, and worked closely with the principal investigators’ research ethics board (REB) to ensure that recruitment and data acquisition procedures were in accordance with current ethical standards.
In April 2016, the Research Group launched a 24-month pilot study to test the proposed methodology and gather a first set of data on the target population. The aim was to describe older adults with dementia who die in LTC facilities in Quebec, the circumstances surrounding their death, and the quality of their care. Details of the methodology are provided in the subsequent section, followed by an overview of the data collected.
Methodology of the Pilot Study
Study Design and Target Population
The current study employed a mortality follow-back study among residents with dementia who died in a LTC facility located in one of three large regions of Quebec: The Eastern Townships, Capitale-Nationale, and Montreal. The target population has a two-level hierarchical structure, with residents nested within facilities. Eligible facilities had to (1) be part of Quebec’s Health and Social Services Network, (2) have been operating for more than a year, and (3) provide care to residents with dementia until their death. Eligible residents had to have (1) a clinical diagnosis of dementia, (2) been admitted to a participating facility at least 30 days before their death, (3) spent at least 15 of their last 30 days of life in the facility, (4) either died in the facility or spent no more than 3 days before their death in an acute care setting, and (5) been visited at least once during the week preceding their death by a close relative known to the facility. These criteria are similar to those of studies conducted in other countries, enabling future international comparisons (Zimmerman et al., Reference Zimmerman, Cohen, van der Steen, van Soest-Poortvliet and Hanson2015).
Recruitment, consent, and data collection procedures were approved by the REB of the CIUSSS de l’Estrie – CHUS (reference # MP-22-2016-576), acting as the designated REB for the ethical approval of studies conducted in more than one healthcare institution (Ministère de la Santé et des Services sociaux, 2016). Prior institutional authorization to conduct the research was obtained from each of the integrated university health and social services centres (CIUSSS) with which participating facilities were affiliated.
Recruitment and Data Acquisition Procedures
The research coordinator reached out by phone to the manager of a potentially eligible facility (or to a substitute locally designated to oversee research activities in the facility), briefly describing the study and soliciting the participation of facilities found eligible. Those willing to engage in the pilot study were met by the research coordinator, who provided more details about the study, showed them the questionnaires we had developed to collect the data, and obtained their written consent to participate. Managers were then asked to fill out a secured online questionnaire on the facility, accessible through a personalized code, and to designate a contact person with whom the research coordinator would interact throughout the data collection period.
The role of the contact person was, first, to consecutively identify residents meeting our eligibility criteria until the target sample size (set at 10 deceased residents per site) or the study end date (March 2018) was reached. For each of these residents, the contact person then had to identify the staff member most involved in the resident’s end-of-life care (often a nurse), as well as the relative who most often visited the resident during his or her last week of life and could communicate in either French or English. The staff member was asked to complete an online questionnaire about the resident within 2 weeks of the resident’s death, so as to minimize recall bias, whereas the relative was mailed an envelope 1 month after the resident had died, followed by a thank you/reminder postcard 2 weeks later. One month was expected to give most relatives enough time to overcome the loss of their loved one, without compromising their ability to remember circumstances surrounding the dying process (Thompson & Chochinov, Reference Thompson and Chochinov2006). The mailed envelope contained a personalized cover letter jointly signed by the principal investigators and the facility manager, a questionnaire that provided consent information on the cover page with a box to tick, and a stamped envelope for returning the questionnaire directly to the research team. Lastly, the contact person gave an experienced research assistant access to the resident’s medical chart for extracting complementary clinical data.
Data for each enrolled resident thus came from four distinct questionnaires, filled out by the resident’s facility manager, a research assistant, the staff member with primary care responsibility for the resident, and the relative most present during the final week of the resident’s life. Variables were included in the questionnaire(s) most likely to maximize data validity while minimizing burden on staff personnel and relatives. An overview of these variables follows.
Variables
The facility manager questionnaire collected information about the facility, including ownership type, size and occupancy rate, care professionals on site for residents with dementia, resources in palliative and end-of-life care, and advance care planning practices. The questionnaire was designed to be updated yearly to capture changes that could affect a facility’s ability to deliver good end-of-life care.
Data abstracted from the medical charts by a research assistant captured clinical information on the resident (e.g., date of admission to the facility, functional autonomy, coexisting medical conditions, primary and contributing causes of death), clinical complications that occurred during the last month of life, interventions performed in response to these complications, and goals of care. Functional autonomy was assessed with the Système de mesure de l’autonomie fonctionnelle (SMAF) (Hébert, Carrier, & Bilodeau, Reference Hébert, Carrier and Bilodeau1988), a validated 29-item scale that generates a total score ranging from 0 (totally independent) to 87 (totally dependent). Coexisting medical conditions were recorded using the Elixhauser Comorbidity Index (Elixhauser, Steiner, Harris, & Coffey, Reference Elixhauser, Steiner, Harris and Coffey1998), modified by van Walraven, Austin, Jennings, Quan, and Forster (Reference van Walraven, Austin, Jennings, Quan and Forster2009) into a single numeric score reflecting the overall burden of illnesses.
Via a secure provincial Internet platform, the designated staff member provided complementary information less likely to be found in medical charts, such as the resident’s capacity to communicate in the week preceding death, and whether decisions were made to withhold or withdraw life-sustaining treatments.
The questionnaire mailed to the close relatives collected socio-demographic information about themselves and the resident, as well as information on the resident’s end-of-life care wishes (e.g., were they known? were they considered in decision making?). Relatives were then invited to complete four assessment tools pertaining to the last month or week of the resident’s life. Two of these tools – Satisfaction With Care (SWC) (Volicer, Hurley, & Blasi, Reference Volicer, Hurley and Blasi2001) and Family Perceptions of Physician-Family Caregiver Communication (FPPFC) (Biola et al., Reference Biola, Sloane, Williams, Daaleman, Williams and Zimmerman2007) – focus on quality of end-of-life care, whereas the other two – Symptom Management (SM) and Comfort Assessment in Dying (CAD) (Volicer et al., Reference Volicer, Hurley and Blasi2001) – focus on the quality of dying. These tools were developed specifically to retrospectively assess quality of end-of-life care and quality of dying among LTC residents with dementia. They have well-established metric properties (Kiely, Shaffer, & Mitchell, Reference Kiely, Shaffer and Mitchell2012; Kiely et al., Reference Kiely, Volicer, Teno, Jones, Prigerson and Mitchell2006; van Soest-Poortvliet et al., Reference van Soest-Poortvliet, van der Steen, Zimmerman, Cohen, Klapwijk and Bezemer2012, Reference van Soest-Poortvliet, van der Steen, Zimmerman, Cohen and Achterberg2013) and have been used extensively in other countries (Cohen et al., Reference Cohen, van der Steen, Reed, Hodgkinson, van Soest-Poortvliet and Sloane2012; van Uden et al., Reference van Uden, den Block, van der Steen, Onwuteaka-Philipsen, Vandervoort and Vander Stichele2013). The four assessment tools were professionally translated into French prior to their use in the pilot study.
The SWC assesses the relative’s satisfaction with the care provided to the resident during his or her last month of life. It consists of 10 items addressing decision making, communication with health care providers, understanding of the resident’s condition, and appropriateness of the care provided to the dying person. Items are rated on a four-point Likert scale, from strongly agree to strongly disagree. Total scores range from 10 to 40, with higher scores reflecting greater satisfaction.
Using the same response scale and time window as the SWC, the seven-item FPPFC assesses whether the family was kept informed, received information about what to expect, understood what the physician was saying, discussed wishes for medical treatment, had the opportunity to ask questions, felt listened to, and felt understood. Total scores range from 0 to 21, with higher scores reflecting greater satisfaction with communication. For the present study, the word “physician” was replaced by “health care team” in all seven items.
The SM quantifies the frequency (from never to every day) with which a resident exhibited nine physical or psychological signs of distress (e.g., shortness of breath, agitation, resistance to care) during the month preceding his or her death. Total scores range from 0 to 45, with higher scores indicating better symptom control.
The CAD assesses the intensity (from not at all to a lot) of 14 symptoms during the last week of life (e.g., pain, restlessness, difficulty swallowing). Total scores range from 14 to 42, with higher scores indicating better comfort while dying. The CAD can be filled out by an involved family member or health care provider. For the pilot study, the CAD was included in both the relative and staff questionnaires, allowing their assessments to be directly compared.
Statistical Analysis
In the tables that follow, characteristics of the facilities, deceased residents and designated relatives, are summarized using the mean ± standard deviation (SD) or standard error of the mean (SEM) for continuous variables and number (percentage) for categorical variables. Missing data on any of the four assessment tools were imputed with the resident’s mean, provided at least two thirds of the items had been completed (van Uden et al., Reference van Uden, den Block, van der Steen, Onwuteaka-Philipsen, Vandervoort and Vander Stichele2013). Using a linear transformation, raw scores were converted to a common scale, ranging from 0 (worst outcome) to 100 (best outcome). This allowed ratings of quality of end-of-life care and quality of dying to be compared visually, using box plots. Analyses were performed with IBM SPSS Statistics, version 25, taking clustering of residents within facilities into account.
Gathering Feedback from Participating Facilities
After closing the pilot study at a given site, the research coordinator contacted the manager one last time to gather feedback on staff’s experience in contributing data to the study, elicit suggestions on how to facilitate recruitment and data collection, and explore their interest in collaborating in future studies conducted within the Observatory.
Findings from the Pilot Study
Facilities Enrolled in the Observatory
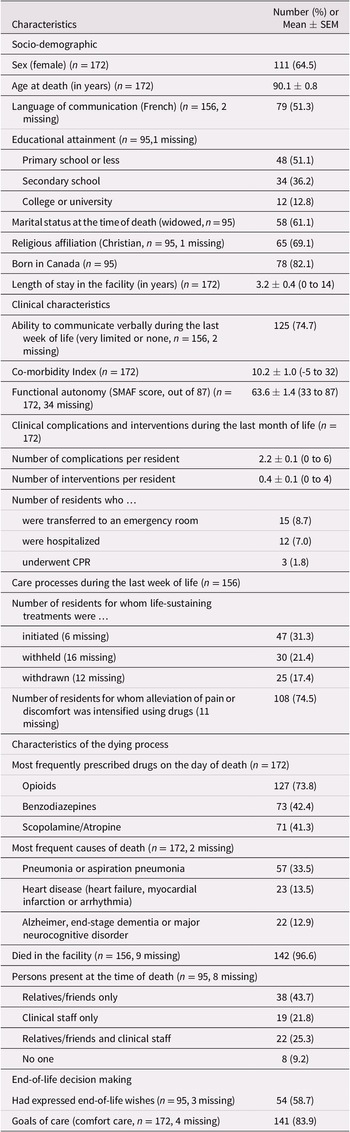
Over the 24-month study period, 16 managers of eligible facilities were invited to join the Observatory, and 13 agreed. Lack of time and human resources to implement the study was the main reason given for refusals. Characteristics of the participating facility are summarized in Table 1. Eleven facilities were public institutions, and two were affiliated with the Jewish community. Bed capacity ranged from 52 to 387 (median 144), with all beds occupied at the time of data collection. Across facilities, from 5 to 90 per cent of residents had mild cognitive impairment (median 15%), and from 10 to 85 per cent had moderate to severe cognitive impairment (median 70%). Availability of care professionals for residents with dementia varied greatly across facilities, except for nursing staff, who were present at all sites. All facilities had in-house personnel trained in palliative and/or end-of-life care, although to varying degrees. According to facility managers, goals of care are established for every newly admitted resident, and are revised following changes in the resident’s condition or at the request of the resident or his/her representative.
Table 1. Characteristics of the 13 participating LTC facilities
Note. LTC = long-term care; SD = standard deviation.
Deceased Residents Enrolled in the Pilot Study
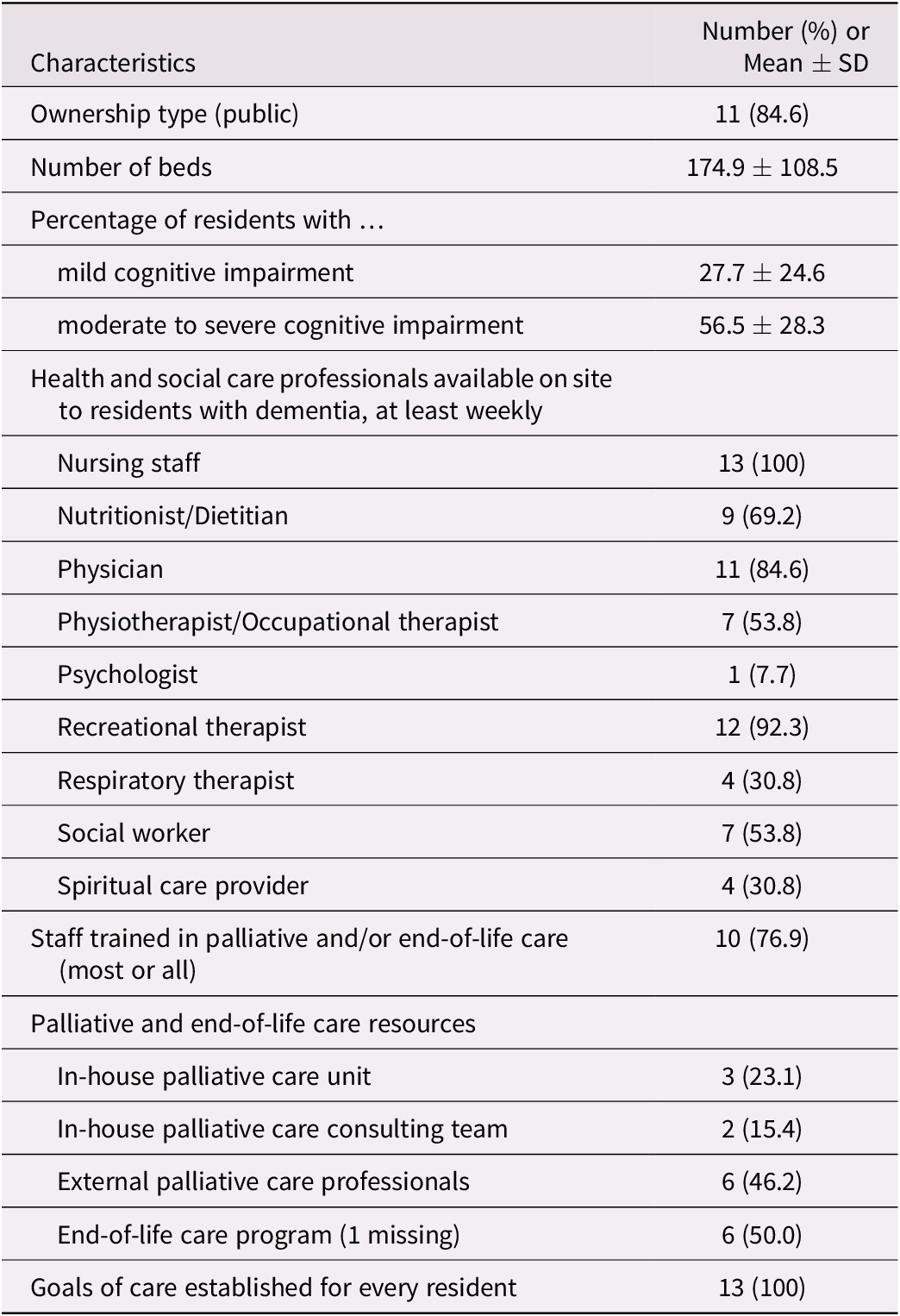
By the end of the data collection period, seven sites had reached the agreed-upon target of 10 deceased residents, and four had enrolled between 3 and 9 participants. Two larger facilities offered to surpass the target and enrolled 24 and 50 participants each, for a total of 172 deceased residents. Their characteristics are displayed in Table 2. Almost two thirds were female, with age at death ranging from 60 to 105 years. On average, the residents had spent 3 years in the facility before their death, with some having been admitted more than 10 years earlier. Most had limited ability to communicate near death and had high levels of co-morbidity, with hypertension, cardiac arrhythmias, and renal failure being among the most prevalent co-morbid conditions. Residents were highly dependent on others for their daily activities, according to their SMAF total score which, on average, had been established 6 months before death.
Table 2. Characteristics of the 172 deceased residents enrolled in the pilot study a
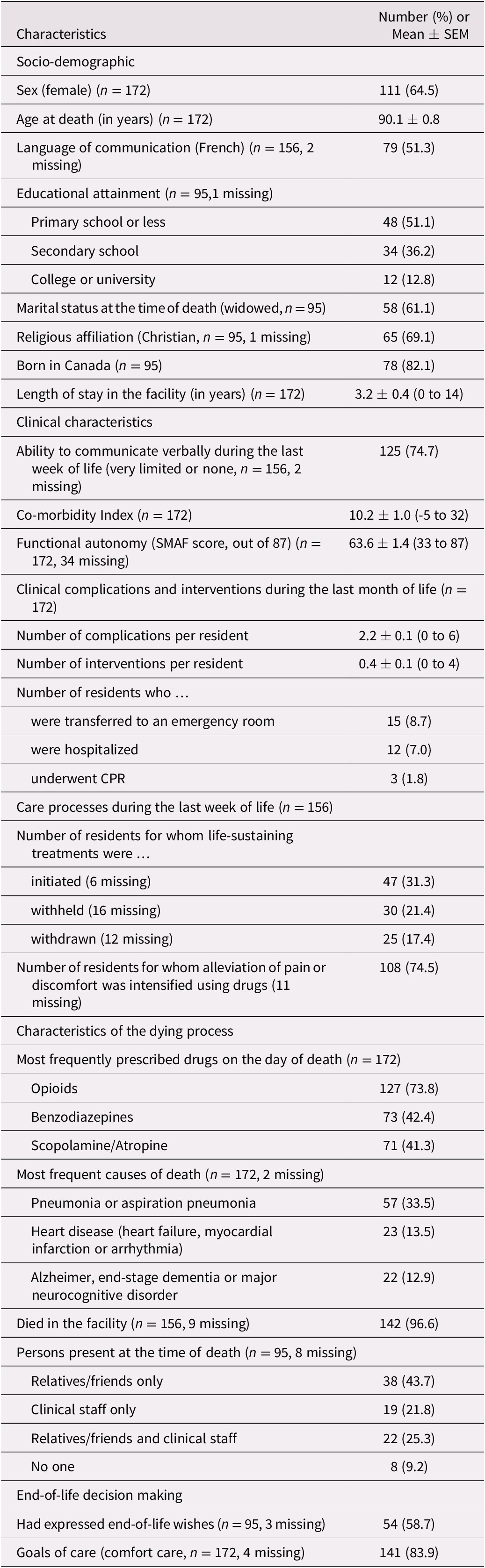
Note. a Maximum sample sizes vary according to the source of the data: designated relatives (n = 95), designated staff members (n = 156), and medical charts (n = 172). These are specified next to each variable, followed by the number of missing data, if any.
SEM = standard error of the mean; SMAF = Système de mesure de l’autonomie fonctionnelle; CPR = cardiopulmonary resuscitation.
Medical charts revealed that residents experienced two clinical complications, on average, during their last month of life (e.g., respiratory distress, dietary intake problems, pneumonia), leading to an average of 0.4 interventions per resident. Few residents were transferred to an emergency room or hospitalized to manage complications. Three residents underwent cardiopulmonary resuscitation (CPR), of whom two died on the same day and one died 2 weeks later.
During the last week of life, interventions to postpone death were initiated in 47 residents, mainly antibiotics and intravenous fluids. These two interventions, along with artificial nutrition and hydration, were also those most often withheld or withdrawn to avoid prolonging life. Alleviation of pain or discomfort was increased using drugs in 75 per cent of residents. Drugs used were opioids in all but three cases, with scopolamine/atropine and benzodiazepines being other drugs used. Many residents were still receiving these drugs on the day of their death. Based on the death certificates, the three most frequent causes of death were pneumonia/aspiration pneumonia, a cardiac problem, or dementia itself. Almost all residents died in their facility, surrounded by close relatives or friends, clinical staff members, or both. Eight residents died alone.
According to the designated relatives, 54 deceased residents (59%) had expressed their wishes regarding end-of-life care, most often in writing. Expressed wishes were judged to have influenced the care provided to the resident in 43 cases (80%). Goals of care were found in all but 4 of the 172 medical charts reviewed, with comfort care most often selected.
Relatives who Returned the Questionnaire
By the end of the pilot study, 95 designated relatives had returned their questionnaire, for an overall response rate of 55 per cent (from 33% to 100% across facilities; median 60%). Their characteristics are presented in Table 3. The majority were female, offspring of the deceased resident, and well educated, with 62 per cent holding at least a college degree. The age range was wide, from 39 to 87 years. Half of the relatives had visited the decedent more than 10 times in the month preceding the resident’s death, and 52 per cent were present at the time of death. More than 70 per cent of relatives had discussed end-of-life care with a nurse, and 63 per cent had discussed this with a physician. One third had also discussed this issue with other family members.
Table 3. Characteristics of the 95 designated relatives who returned the questionnaire
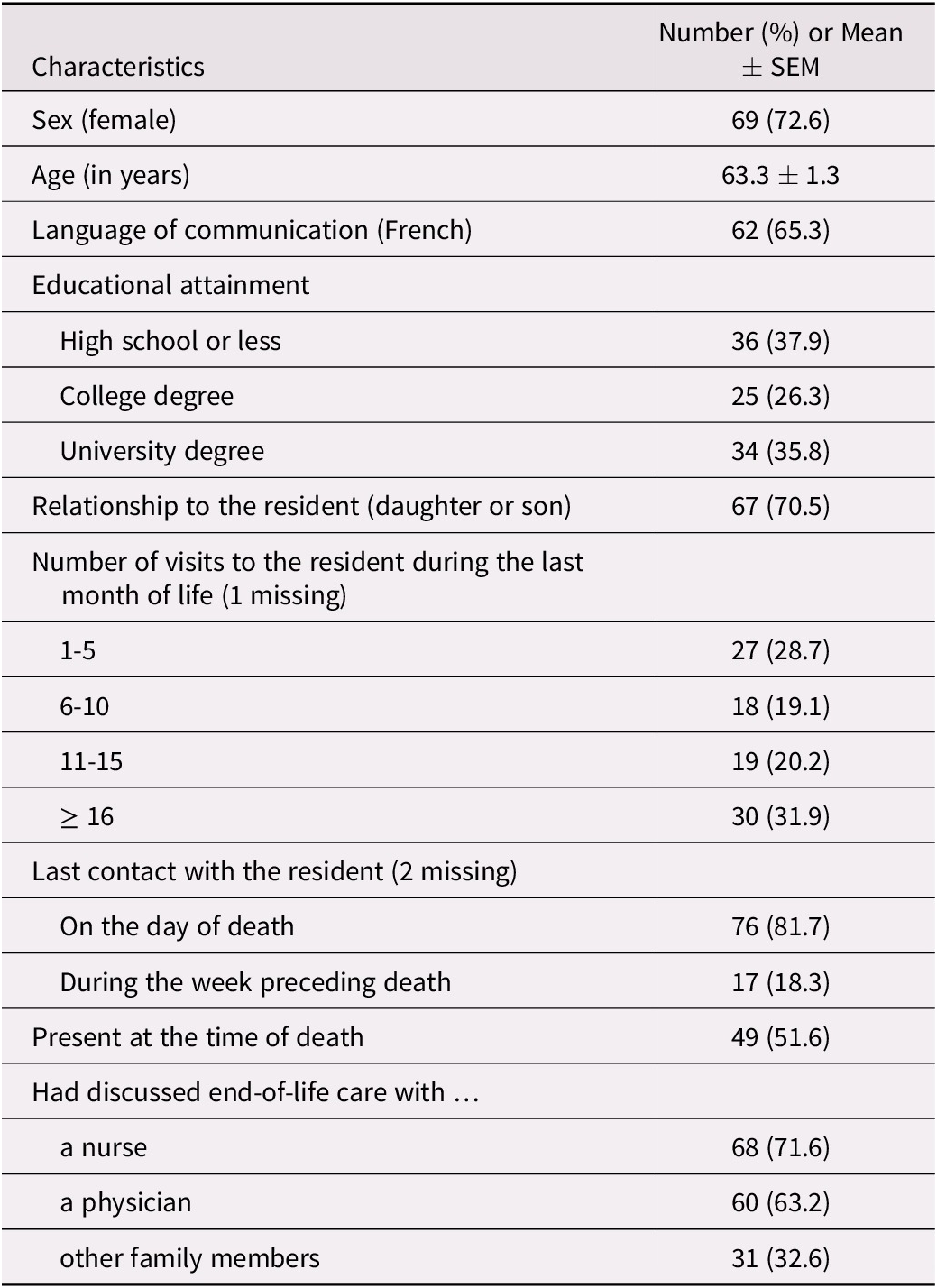
Note. SEM = standard error of the mean.
Quality of End-of-Life Care and Quality of Dying, According to Designated Relatives and Staff Members
Means and associated 95 per cent confidence intervals for the four assessment tools are depicted in Figure 1. Means derived from relatives’ ratings were relatively high for the SWC and FPPFC, at 78.8 and 78.3, respectively. By comparison, SM ratings were lower (mean 62.4), and lower still for the CAD (mean 56.4). Relatives’ assessments of decedents’ comfort levels were lower, on average, than those provided by designated staff members: means of 56.4 versus 65.5 on the CAD, p = 0.027.
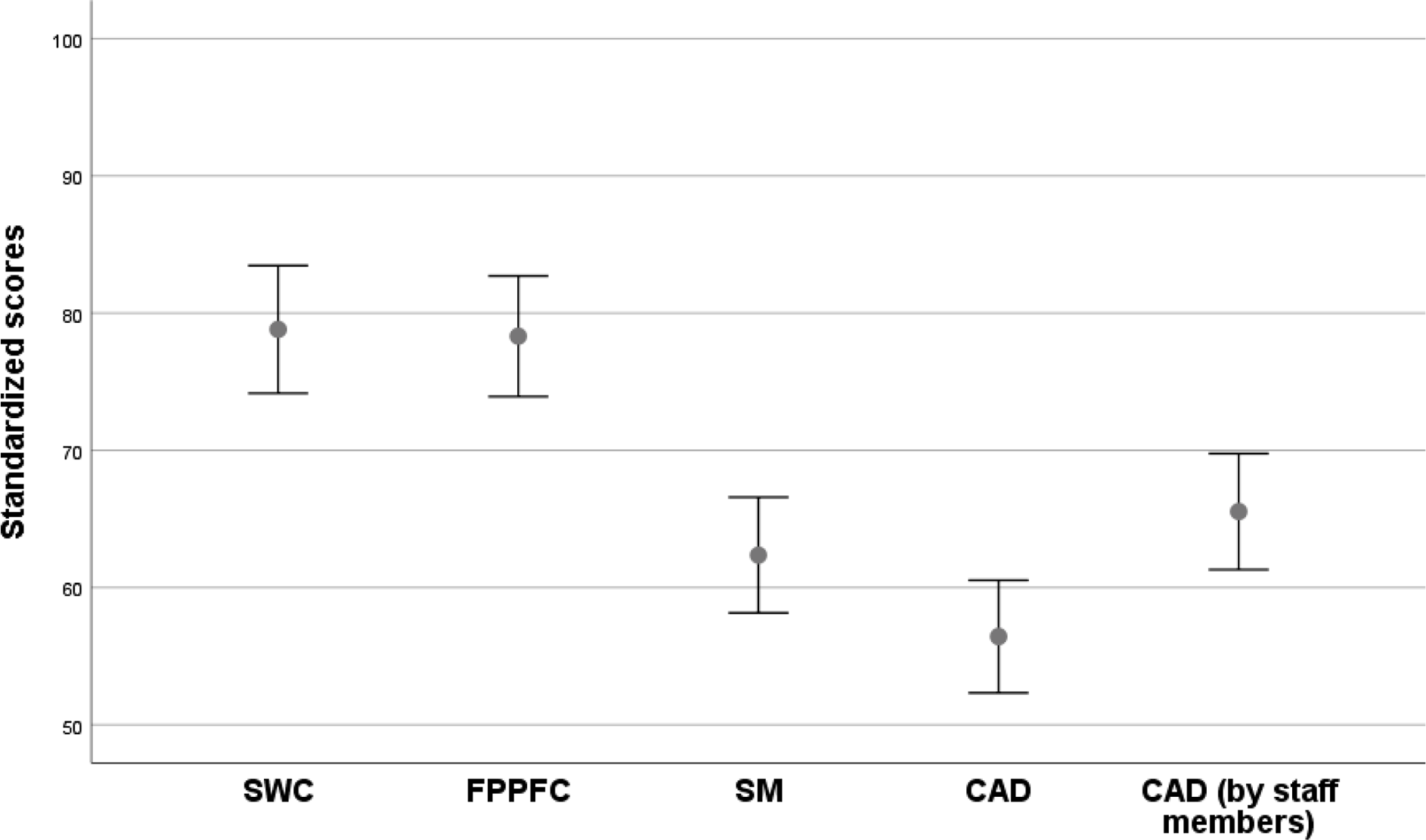
Figure 1. Box plots (mean ± 1.96 SEM) for each of the four assessment tools, with scores converted to a common scale from 0 (worst outcome) to 100 (best outcome). The four assessment tools were completed by relatives, with the CAD also completed by staff members. SWC = Satisfaction with Care; FPPFC = Family Perceptions of Physician-Family Caregiver Communication; SM = Symptom Management; CAD = Comfort Assessment in Dying
Designated relatives and staff members were also asked to rate the conditions of the resident’s death in general, from very good to very poor, with the option of ticking I prefer not to answer. Distributions of ratings are shown in Figure 2. Approximately 80 per cent of relatives and staff members rated the conditions of death as good or very good, with staff respondents being somewhat more critical than relatives.
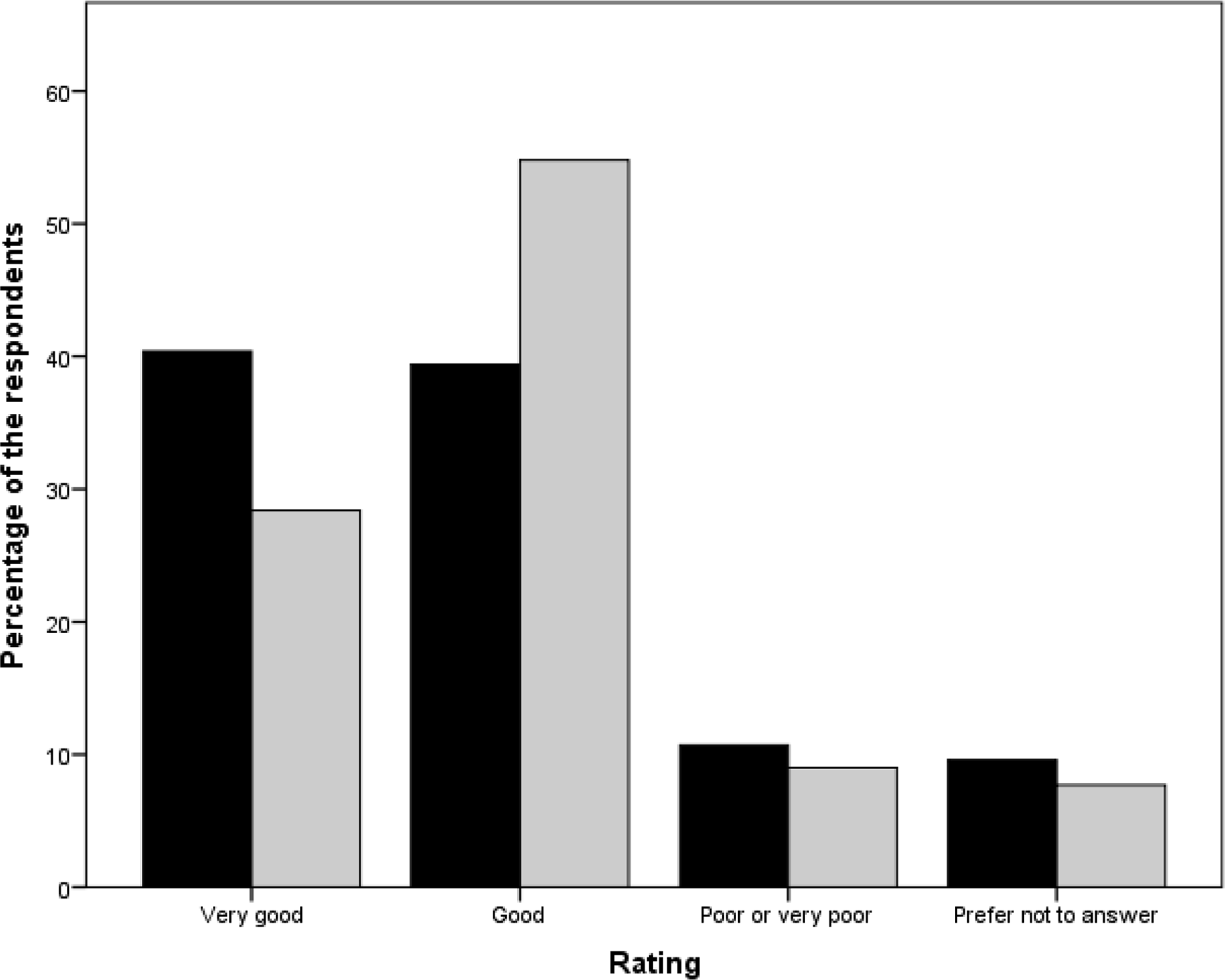
Figure 2. Ratings of the conditions of death, by designated relatives (n = 94, in black) and staff members (n = 155, in grey)
At the very end of their questionnaire, staff members were asked to select, among a list of factors, all those that could have improved the resident’s dying experience. In decreasing order, the factors most often selected were more time/a lower workload (29%), greater family involvement (22%), more human resources (15%), and better medical management (12%).
Changes to Research Procedures as the Pilot Study Unfolded
Some changes were made to research procedures shortly after launching the study. These were allowing two staff members to share the task of providing data on a given resident, moving some variables to a different questionnaire for greater validity, and deactivating the online version of the questionnaire designed for staff members because of their limited computer access. Additionally, some sites adapted procedures to their own environment or culture. For example, one preferred to inform families of the study when it was judged that their loved one with dementia was entering the final phase, rather than after death had occurred.
Feedback from Facility Managers
Feedback from managers elicited after closing data collection at their site was positive. Although some had initially raised concerns; for example, about anonymity of the data to be published subsequently, the legality of contacting relatives of decreased residents, and lack of resources to support the study, in the end all felt that the recruitment and data collection procedures had been relatively easy to implement, and that they were neither labour intensive nor time consuming. The small number of residents to enroll (10 for most sites) contributed to the managers’ positive experience.
Some managers proposed additions to the questionnaires; for example, asking relatives whether they feel that the health care team needs to be better trained, and if so, on what? Another manager with a strong interest in spiritual care suggested collecting data on interventions aimed at addressing residents’ spiritual needs. Several managers asked for a summary of study findings that would be specific to their own facility, along with comparative data from the other (anonymized) sites. All were willing to collaborate in future studies conducted within the Observatory.
Discussion
Implementing the Quebec Observatory
Morley et al. (Reference Morley, Caplan, Cesari, Dong, Flaherty and Grossberg2014) conducted a survey among 19 experts from eight countries, including Canada, to identify research priorities for nursing homes. The most important areas for research were related to the needs of cognitively impaired residents, with 3 of the top 15 priorities focused on end-of-life care. A more recent priority-setting exercise for LTC research, conducted in Alberta with persons living with dementia, caregivers of LTC residents, and other stakeholders, identified the determinants of resident outcomes as top research priorities (Chamberlain et al., Reference Chamberlain, Estabrooks, Keefe, Hoben, Berendonk and Corbett2020). The Quebec Observatory on End-of-Life Care for People with Dementia is focused on addressing these research priorities, in partnership with LTC settings.
The experience of implementing the Quebec Observatory was generally positive. Targeted facilities responded positively to the invitation to join the Observatory and were willing to pursue collaboration in the future. Participation rates among facilities (13 out of 16) and designated staff (156/172) were higher than most reported so far, whereas that among relatives (95/172) was similar (Kiely et al., Reference Kiely, Shaffer and Mitchell2012; Lee et al., Reference Lee, Sussman, Kaasalainen, Durepos, McCleary and Wickson-Griffiths2020; van der Steen, Ribbe, Deliens, Gutschow, & Onwuteaka-Philipsen, Reference van der Steen, Ribbe, Deliens, Gutschow and Onwuteaka-Philipsen2014; Vandervoort et al., Reference Vandervoort, Van den Block, van der Steen, Volicer, Vander Stichele and Houttekier2013). Importantly, all consecutive deaths of residents with dementia were included, irrespective of staff or family participation afterward, thus assembling a well-defined and representative sample of residents who actually died (Earle & Ayanian, Reference Earle and Ayanian2006; Thompson & Chochinov, Reference Thompson and Chochinov2006; van der Steen et al., Reference van der Steen, Ribbe, Deliens, Gutschow and Onwuteaka-Philipsen2014).
The questionnaires used for data collection proved effective, although some variables had considerable missing data. These include the SMAF, a measure of functional autonomy that is mandatory in Quebec, yet which could not be found in 34 of the 172 medical charts reviewed. Data on whether treatments were withheld or withdrawn, and on drugs used to alleviate pain and discomfort at end of life, were also missing for several residents. This was information that designated staff members were expected to be able to provide. Lastly, significant numbers of SM and CAD assessments were left incomplete, by both designated relatives and staff, suggesting that it may have been difficult for them to provide reliable responses to some scale items.
Future Plans
The Observatory was successfully implemented, but it was restricted to facilities located in urban areas. While data collection in participating facilities continues to reach larger sample sizes, recruitment will be expanded to sites located in more remote areas. Expanding the Observatory to other Canadian provinces and territories would also be important, enabling interprovincial/territorial comparisons. Costs associated with collecting data from facility managers, staff members, and close relatives would not be much impacted by such expansions, as questionnaires are returned electronically or by mail. Chart abstraction, however, would require identifying (and perhaps funding) qualified persons on site to extract the data. An alternative to chart abstraction is linkage of the data collected with health administrative databases and other sources of “big” data (Anderson & Oderkirk, Reference Anderson and Oderkirk2015). Although promising, this strategy would likely be difficult to implement across the country, given marked differences in provincial database contents. Research in the LTC setting has been conducted in Canada (e.g., Boscart et al., Reference Boscart, Sidani, Poss, Davey, d’Avernas and Brown2018; Estabrooks et al., Reference Estabrooks, Hoben, Poss, Chamberlain, Thompson and Silvius2015; Xiong, Freeman, Banner, & Spirgiene, Reference Xiong, Freeman, Banner and Spirgiene2019) and elsewhere (e.g., Mitchell et al., Reference Mitchell, Teno, Kiely, Shaffer, Jones and Prigerdson2009) using interRAI assessments. As the interRAI is not implemented in Quebec, it cannot be a source of comparative data across the country that includes Quebec.
In the near future, an advisory committee will be set up that is representative of relevant stakeholder groups (e.g., still-competent LTC residents with dementia, formal and informal dementia caregivers, facility managers) to promote effective knowledge transfer of future study findings (Chamberlain et al., Reference Chamberlain, Estabrooks, Keefe, Hoben, Berendonk and Corbett2020; Keefe et al., Reference Keefe, Hande, Aubrecht, Daly, Cloutier and Taylor2020). Secondary analyses will also be performed on the rich set of pilot data accumulated so far. Planned analyses include establishing the degree of congruence between goals of care and care actually delivered, identifying correlates of better resident outcomes, and studying individual items of the assessment tools to better pinpoint areas for improvement.
No data were collected on designated staff members, and only socio-demographic data were collected on designated relatives. Although this was justified by the need to keep questionnaires as short as possible, staff members and relatives have needs of their own worth studying. Future studies conducted within the Observatory could investigate, for example, staff working conditions and needs for emotional support (Vandrevala et al., Reference Vandrevala, Samsi, Rose, Adenrele, Barnes and Manthorpe2017). Bereaved relatives’ ability to cope with the loss of a loved one would also be worth exploring. Additionally, attention could be given to the spiritual needs of residents and families and the quality of spiritual care, two important but under-studied areas (Palmer, Smith, Paasche-Orlow, & Fitchett, Reference Palmer, Smith, Paasche-Orlow and Fitchett2020).
Limitations
The portrait drawn from the pilot data must be interpreted with the consideration that there were some limitations. Data were collected retrospectively, which increases recall bias compared with prospective approaches (Earle & Ayanian, Reference Earle and Ayanian2006; Thompson & Chochinov, Reference Thompson and Chochinov2006; van der Steen et al., Reference van der Steen, Ribbe, Deliens, Gutschow and Onwuteaka-Philipsen2014). This bias was countered by minimizing the time lapse between the resident’s death and distribution of the questionnaires, and by focusing on the last month and week of life. Moreover, data originate from different sources, thus avoiding biases inherent in collecting data exclusively from facility staff. Validated tools were employed to measure important constructs, such as satisfaction with care and level of comfort, but proxy ratings may be influenced by several factors (e.g., low expectation of care, reluctance to criticize service providers) and not reflect actual care (Thompson & Chochinov, Reference Thompson and Chochinov2006). Despite these limitations, proxy assessments of quality of end-of-life care and quality of dying are considered valuable alternatives when patients cannot provide such assessments themselves.
Conclusion
End-of-life care for people with dementia is difficult to investigate because of the many methodological challenges, but is critical to study in order to identify deficiencies in care quality, their impact on resident outcomes, and areas for improvement (Estabrooks et al., Reference Estabrooks, Straus, Flood, Keefe, Armstrong and Donner2020). To this end, the first Observatory on End-of-Life Care for People with Dementia was successfully implemented in Quebec, and pilot data were used to draw a preliminary portrait of this population. Continuing data acquisition, in currently involved facilities and others, will provide larger sample sizes for subgroup analyses and allow variation in care over time to be investigated. The COVID-19 pandemic has exposed long-standing and widespread deficiencies in LTC, and has exacerbated the need to reform Canada’s LTC sector (Béland & Marier, Reference Béland and Marier2020; Canadian Institute for Health Information, 2021; Estabrooks et al., Reference Estabrooks, Straus, Flood, Keefe, Armstrong and Donner2020; Holroyd-Leduc & Laupacis, Reference Holroyd-Leduc and Laupacis2020; Marrocco, Coke, & Kitts, Reference Marrocco, Coke and Kitts2021). The newly implemented Observatory could prove useful for assessing the impact of urgently needed LTC reforms and the effects of innovative interventions in dementia end-of-life care, for which the evidence base is currently very limited (Sampson et al., Reference Sampson, Candy, Davis, Gola, Harrington and King2018).
Acknowledgments
The authors thank Claire-Marie Legendre, Lucie Misson, Marie-Claire Chalifoux, Stephanie Ballard, Brandon Asimov, Kayte Andersen, and Ivo Ivanov for their assistance with data collection at study sites. We also thank the 13 individuals who agreed to their LTC facilities’ being part of the Observatory, as well as staff and family members who contributed data on deceased residents.
Funding
Financial support for implementing the Observatory and conducting the pilot study was provided by the Research Centre on Aging of the CIUSSS de l’Estrie – CHUS, Vitae Foundation, the Quebec Network for Research on Aging, and the Donald Berman Maimonides Medical Research Foundation.
Appendix
Additional Members of the End-of-Life Care in Dementia Research Group
Nathalie Champoux, Department of Family Medicine and Emergency Medicine, Faculty of Medicine, Université de Montréal, Montreal, Quebec.
Guillaume Léonard, School of Rehabilitation, Faculty of Medicine and Health Sciences, Université de Sherbrooke, and Research Centre on Aging, CIUSSS de l’Estrie – CHUS, Sherbrooke, Quebec.
Véronique Provencher, School of Rehabilitation, Faculty of Medicine and Health Sciences, Université de Sherbrooke, and Research Centre on Aging, CIUSSS de l’Estrie – CHUS, Sherbrooke, Quebec.