Introduction
Parkinsonism is a leading cause of neurological disability worldwide, associated with impaired work capacity, quality of life, and survival.Reference Koerts, Konig, Tucha and Tucha1–Reference Macleod, Taylor and Counsell4 Parkinsonism is also costly to society.Reference Koerts, Konig, Tucha and Tucha1, Reference Rodriguez-Blazquez, Forjaz, Lizan, Paz and Martinez-Martin5–Reference Kowal, Dall, Chakrabarti, Storm and Jain7 Since parkinsonism can affect younger adults, understanding the timing and trends of disease onset (young vs. mid/late-onset) has important clinical implications on etiology and prognosis.8, Reference Grimes, Gordon and Snelgrove9 Genetic forms of parkinsonism (~2% of sporadic, ~10% of familial cases) would more likely present in younger patients and be less prone to change over time.Reference Marras, Lang and van de Warrenburg10, Reference Schiesling, Kieper, Seidel and Kruger11
The World Health Organization emphasized a need for up-to-date information about the burden of parkinsonism and Parkinson’s disease.2, 8 However, studies have found different trends in the incidence of parkinsonism and Parkinson’s disease. For Parkinson’s disease, two studies reported relatively stable incidence,Reference Akushevich, Kravchenko, Ukraintseva, Arbeev and Yashin12, Reference Lix, Hobson, Azimaee, Leslie, Burchill and Hobson13 three studies reported decreasing incidence,Reference Rocca, Bower, McDonnell, Peterson and Maraganore14–Reference Darweesh, Koudstaal, Stricker, Hofman and Ikram16 and two studies reported increasing incidence.Reference Liu, Wu, Lin, Liu, Chang and Lin17, Reference Savica, Grossardt, Bower, Ahlskog, Mielke and Rocca18 For parkinsonism, one study reported relatively unchanged incidence trends over time,Reference Savica, Grossardt, Bower, Ahlskog and Rocca19 while one study reported decreasing incidence.Reference Ylikotila, Tiirikka, Moilanen, Kääriäinen, Marttila and Majamaa20 Less is known about the prevalence and mortality trends of parkinsonismReference Goldacre, Duncan, Griffith and Turner21 or Parkinson’s disease.Reference Lix, Hobson, Azimaee, Leslie, Burchill and Hobson13, Reference Darweesh, Koudstaal, Stricker, Hofman and Ikram16, Reference Guttman, Slaughter, Theriault, DeBoer and Naylor22–Reference Griffiths and Rooney24 Since most studies analyzed data collected prior to 2010,Reference Darweesh, Koudstaal, Stricker, Hofman and Ikram16, Reference Goldacre, Duncan, Griffith and Turner21–Reference Mylne, Griffiths, Rooney and Doyle23 whether these trends persist in recent years remains unknown. It has been hypothesized that parkinsonism incidence is decreasing while prevalence is increasing over time, similar to trends for other neurodegenerative pathologies.Reference Jaakkimainen, Bronskill and Tierney25
Understanding the incidence and mortality of parkinsonism will enable quantification of disease burden to establish priorities. Characterizing the prevalence of pre-existing conditions as accepted or suspected risk factors will further inform future hypotheses on the development of parkinsonism. Thus, we conducted a large population-based study to determine time trends of the incidence, prevalence, and post-diagnosis mortality of parkinsonism in Ontario, Canada from 1996 to 2014. We also explored the influence of risk factors for brain health on trends of incident parkinsonism during this period.
Methods
Study Design and Population
We established a retrospective cohort in Ontario, Canada by linking population-based health administrative and vital statistics databases using unique encoded identifiers and analyzed at ICES. This cohort included all Ontario residents who, from 1991 to 2013 fiscal years (i.e., April 1, 1991 to March 31, 2014), were (1) aged 20–100 years, (2) registered with the provincial health insurance plan (Ontario Health Insurance Plan (OHIP)) for at least 5 years, and (3) diagnosed with incident parkinsonism.
Standard Protocol Approvals, Registrations, and Patient Consents
Ethical approval for the study was obtained from the Sunnybrook Health Sciences Centre Research Ethics Board (#159-2014). The full dataset creation plan is available from the authors upon request.
Data Sources
We assembled this cohort using the Ontario’s Registered Persons Database, a registry of all Ontario residents who have ever had provincial health insurance.Reference Chan26 In addition, we used the following databases to ascertain incident cases of parkinsonism: (1) OHIP for physician billing claims and (2) Ontario Drug Benefits (ODB) for prescription medication claims (for those aged 65 and older). To obtain information on demographics and risk factors (accepted or suspected) of parkinsonism, we used the following: (1) National Ambulatory Care Reporting System (emergency department data); (2) Canadian Census; and (3) administrative database algorithms for diabetes, congestive heart failure, chronic obstructive pulmonary disease (COPD), traumatic brain injury, dementia, and hypertension.Reference Chen, Kwong and Copes27–Reference Schultz, Rothwell, Chen and Tu31 Furthermore, we used the Office of the Registrar General Deaths database to obtain death data among persons diagnosed with parkinsonism. These databases cover virtually the entire Ontario population.Reference Chen, Kwong and Copes27
Case Ascertainment for Parkinsonism
To ascertain incident cases of parkinsonism, we applied a validated algorithm to the OHIP and ODB databases.Reference Butt, Tu and Young32 Specifically, parkinsonism was determined based on one physician claim with a diagnostic code for parkinsonism and one drug claim for a medication used to treat parkinsonism within a 6-month period, or two physician claims within a 1-year period. The algorithm was previously validated and shown to have a sensitivity of 78.4%, specificity of 99.9%, positive predictive value of 75.4%, and negative predictive value of 99.9%.Reference Butt, Tu and Young32
Selected Risk Factors of Newly Diagnosed Parkinsonism
To better understand trends in the incidence of parkinsonism (especially mid/late-onset), we selected a wide array of health conditions that are either accepted or suspected risk factors for some forms of parkinsonism.Reference Breckenridge, Berry, Chang, Sielken and Mandel33–Reference Wirdefeldt, Adami, Cole, Trichopoulos and Mandel39 We ascertained all selected medical factors among persons with parkinsonism over a 5-year period before the diagnosis, with the exception of dementia, which was ascertained since 1991.Reference Chen, Kwong and Copes27 To ascertain dementia, diabetes, traumatic brain injury, hypertension, COPD, and congestive heart failure, we used data on hospital discharges, physician office visits, and emergency room visits with administrative database algorithms, as used in previous studies (Table 1).Reference Tu, Chen and Lipscombe28–Reference Schultz, Rothwell, Chen and Tu31 We used hospitalization data to ascertain the presence of stroke and coronary artery disease. Finally, we used survey data from Farm and Food Care Ontario to ascertain pesticide use and the Canadian Community Health Survey to ascertain smoking in the general population.40
Table 1: Administrative database algorithms to ascertain selected risk factors among persons with parkinsonism
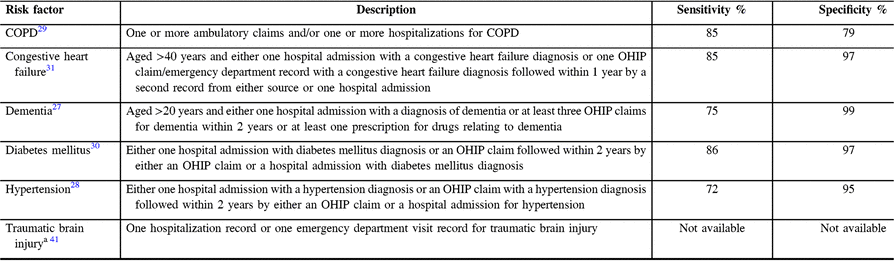
a Considered an accepted algorithm (Evidence Grade II).Reference Ng, Maxwell and Yates41
COPD = chronic obstructive pulmonary disease; OHIP = Ontario Health Insurance Plan.
Analysis
We described the cohort of incident cases of parkinsonism with respect to sex, age at diagnosis, income quintile, residence, and onset of parkinsonism, using means (standard deviations) and percentages to report continuous and dichotomous variables, respectively.
Annual Incidence, Prevalence, and Mortality Rate
We calculated both crude and standardized (by age and sex) incidence, prevalence, and mortality each year between 1996 and 2013 fiscal years (i.e., starting on April 1). To calculate annual incidence, we used newly diagnosed cases of parkinsonism as the numerator and person-years at risk (i.e., excluding prevalent cases) as the denominator. Similarly, we calculated annual prevalence using prevalent cases of parkinsonism who were alive and lived in Ontario at the beginning of each fiscal year as the numerator and the entire Ontario population aged 20–100 years as the denominator. We calculated annual all-cause mortality rate from persons who lived with parkinsonism at the beginning of each year. To obtain age- and sex-standardized incidence and prevalence, we used 2001 Census data for the Ontario population on July 1 of each year.42 We standardized post-diagnosis mortality using all Ontario residents living with parkinsonism in 2005 as the reference. We selected this reference (rather than using 2001 Census data for the Ontario population) because the age and sex distribution of individuals with parkinsonism are distinctly different from the general population. All analyses were conducted by young-onset (aged 20–39 years) and mid/late-onset parkinsonism (aged ≥40 years) separately, according to the World Health Organization.8 We also stratified incidence and prevalence by sex.
Lastly, we estimated the annual prevalence of selected risk factors for brain health (i.e., stroke, traumatic brain injury, diabetes, dementia, hypertension, congestive heart failure, coronary artery disease, and COPD) among persons with a new diagnosis of parkinsonism and among the general population during the study period, respectively.
Time Trend Analysis
To characterize time trends of incidence and mortality of parkinsonism, we used Poisson and negative binomial regression models, respectively. To assess time trends in mortality, we applied negative binomial regression that accounted for zero counts.Reference Liu, Wu, Lin, Liu, Chang and Lin17, Reference Gardner, Mulvey and Shaw43 In all the models, we adjusted for year (continuous variable), age group (ordinal variable: 5-year intervals between 20 and 89 years, and ≥90 years), and sex (categorical variable), with person-years as the offset.
We also used the two-sided Cochran–Armitage trend test to evaluate time trends of the age- and sex-standardized prevalence and the above-mentioned risk factors of parkinsonism over four equal-interval epochs: 1998–2001 (epoch 1), 2002–2005 (epoch 2), 2006–2009 (epoch 3), and 2010–2013 (epoch 4). We computed the average prevalence in each epoch to assess these time trends. Furthermore, we performed Poisson regression modeling to investigate trends in the incidence of mid/late-onset parkinsonism after accounting for prevalence of the above-mentioned risk factors derived in persons with parkinsonism and in the general population, respectively. We also adjusted for rural residence among persons with parkinsonism and the prevalence of smoking and pesticide use in the general population. Statistical significance was set at an alpha level of 0.05.
Results
Characteristics of Incident Parkinsonism
From 1996 to 2014, we identified 73,129 incident cases of parkinsonism in Ontario (population 10.5 million in 2013), with 56% male and a mean age of 72.6 years at diagnosis (Table 2). The proportion of incident cases who were male increased from 53% in 1996/97 to 58% in 2013/14, whereas the mean age at diagnosis remained constant over the time period. Approximately 99% of incident cases had mid/late-onset and 1% had young-onset parkinsonism in 2013/14. The proportion of patients living in higher income neighborhoods increased between 1996/97 and 2013/14.
Table 2: Characteristics of persons with incident parkinsonism (including Parkinson’s disease) aged 20 years and older in Ontario, Canada, from 1996/97 to 2013/14a
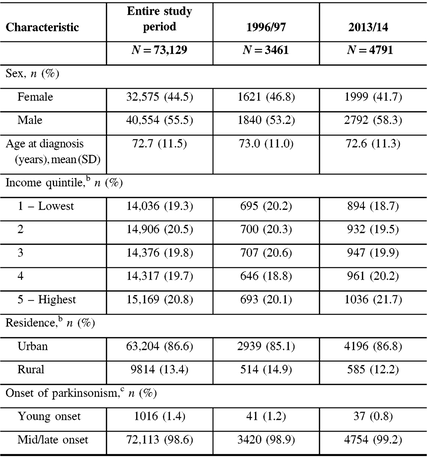
a Fiscal year starts on April 1.
b Percentages do not add up to 100% due to missing values.
c Young onset is 20–39 years of age; mid/late onset is ≥40 years of age.
SD = standard deviation.
Age- and Sex-standardized Incidence, Prevalence, and Post-Diagnosis Mortality of Parkinsonism
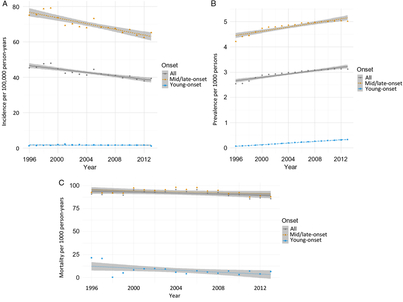
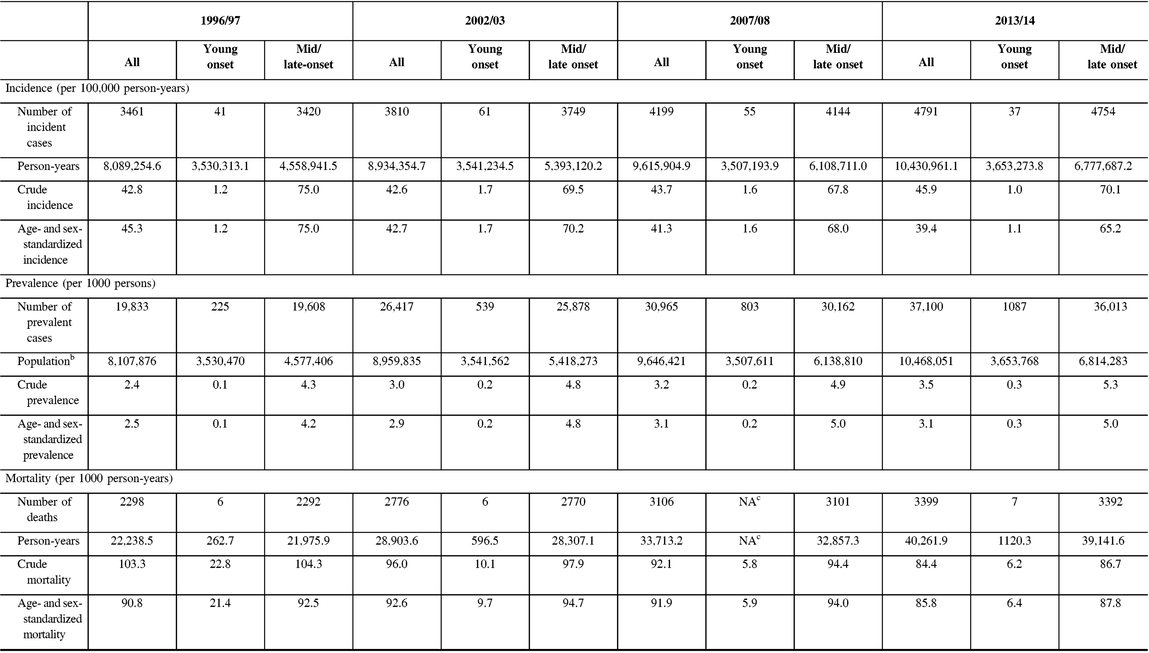
From 1996 to 2014, incidence remained relatively unchanged for young-onset parkinsonism (p = 0.7341) but decreased by 13.1% for mid/late-onset parkinsonism (p < 0.0001) (Table 3; Figure 1A). When stratified by sex, the incidence of all parkinsonism decreased by 9.3% for males (p < 0.0001) and 17.5% for females (p < 0.0001).
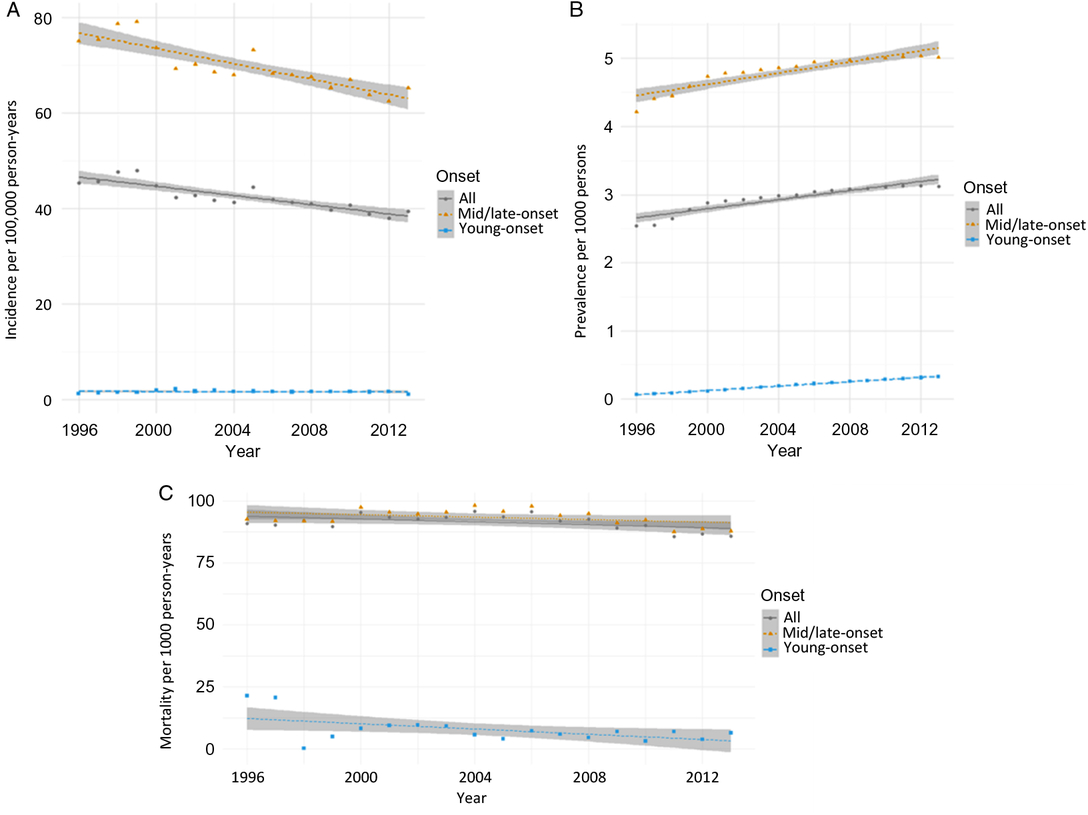
Figure 1: Age- and sex-standardized (incidence and prevalence are standardized using the 2001 Census data for the Ontario population; mortality is standardized using the 2005 data for the prevalent parkinsonism cases in Ontario) incidence (A), prevalence (B), and post-diagnosis mortality (C) of parkinsonism from 1996/97 to 2013/14, by young onset (20–39 years) and mid/late onset (≥40 years) (Figure 1A (incidence): all, p < 0.0001; young onset, p = 0.7341; mid/late onset, p < 0.0001. Figure 1B (prevalence): all, p < 0.0001; young onset, p < 0.0001; mid/late onset, p < 0.0001. Figure 1C (mortality i.e., all deaths among persons with parkinsonism): all, p = 0.0180; young onset, p = 0.0024; mid/late onset, p = 0.0283).
Table 3: Crude, and age- and sex-standardizeda incidence, prevalence, and post-diagnosis mortality parkinsonism (including Parkinson’s disease) in 1996/97, 2002/03, 2007/08, and 2013/14b, by young-onset (20–39 years) and mid/late onset (≥40 years)
a Incidence and prevalence are standardized using the 2001 Census data for the Ontario population; mortality is standardized using the 2005 data for the prevalent parkinsonism cases in Ontario.
b Fiscal year starts on April 1.
c NA = not available due to cell sizes ≤5 (not reported to protect confidentiality of subjects).
Prevalence increased by 22.8% over the 18 years (p < 0.0001), with similar increasing trends among those with young-onset and mid/late-onset parkinsonism (Table 3; Figure 1B). The prevalence of parkinsonism increased by 21.9% for males (p < 0.0001) and 23.8% for females (p < 0.0001).
Mortality rate among individuals living with parkinsonism decreased by 5.5% (p = 0.0180) over the 18 years, with similar decreasing trends for both young-onset and mid/late-onset parkinsonism (Table 3; Figure 1C).
Age- and Sex-Standardized Prevalence of Risk Factors among Persons with Parkinsonism
Among individuals with newly diagnosed parkinsonism, the prevalence of the following risk factors decreased over the four epochs: COPD (−53.0%; p < 0.0001), stroke (−51.2%; p = 0.0008), coronary artery disease (−41.7%; p = 0.0265), and congestive heart failure (−27.6%; p = 0.0027) (Figure 2). There was no statistically significant decrease in the prevalence of traumatic brain injury. Conversely, the prevalence of diabetes increased by 99.3%, dementia increased by 31.0%, and hypertension increased by 29.5% (p < 0.0001) over the four epochs. Further adjustment for these risk factors among persons with parkinsonism and the general population had little influence on the declining trend in the incidence of mid/late-onset parkinsonism (Supplementary File). Similarly, adjustment for rural living among persons with parkinsonism and prevalence of other factors (smoking and pesticide use) among the general population had little influence on the declining incidence of mid/late-onset parkinsonism (Supplementary File).
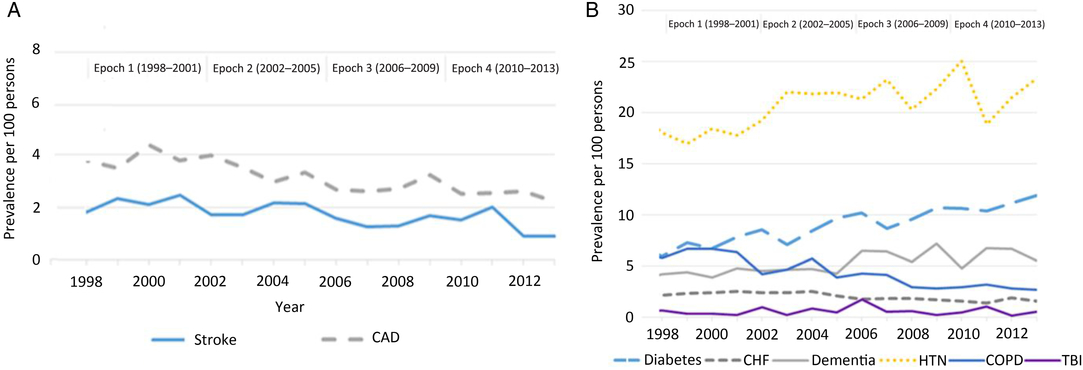
Figure 2: Age- and sex-standardized (standardized using the 2001 Census data for the Ontario Population) prevalence of risk factors ascertained using CIHI hospitalization data (A) and algorithms (B) among persons with parkinsonism from 1998/99 to 2013/14 (Figure 2A: coronary artery disease, p = 0.0265; stroke, p = 0.0008. Figure 2B: congestive heart failure, p = 0.0027; COPD, p < 0.0001; dementia, p < 0.0001; diabetes, p < 0.0001; hypertension, p < 0.0001; traumatic brain injury, p = 0.3047). Acronyms: CAD = coronary artery disease; CHF = congestive heart failure; CIHI = Canadian Institute for Health Information; COPD = chronic obstructive pulmonary disease; HTN = hypertension; TBI = traumatic brain injury.
Discussion
In Ontario, Canada’s most populous province, the age- and sex-standardized incidence of parkinsonism decreased, prevalence increased, and mortality decreased from 1996 to 2014. However, there were differing rates in incidence when stratified by young-onset and mid/late-onset parkinsonism. The incidence for mid/late-onset parkinsonism decreased over time but was unchanged for young-onset parkinsonism, as might be expected for a group more likely to have genetic parkinsonism.Reference Marras, Lang and van de Warrenburg10, Reference Schiesling, Kieper, Seidel and Kruger11 Adjustment for a number of risk factors for brain health conditions did not account for the declining trend in incidence of mid/late-onset parkinsonism.
Several previous studies have examined the trends of incidence in parkinsonism. Among them, three studies reported relatively stable incidence rates in the USA and Manitoba, Canada, but two reported increasing incidence rates in the USA and Finland.Reference Akushevich, Kravchenko, Ukraintseva, Arbeev and Yashin12, Reference Lix, Hobson, Azimaee, Leslie, Burchill and Hobson13, Reference Liu, Wu, Lin, Liu, Chang and Lin17–Reference Savica, Grossardt, Bower, Ahlskog and Rocca19 We found decreasing trends in the incidence of mid/late-onset parkinsonism, which provides support to four other smaller studies suggesting decreasing incidence rates in USA, UK, the Netherlands, and Taiwan.Reference Rocca, Bower, McDonnell, Peterson and Maraganore14–Reference Darweesh, Koudstaal, Stricker, Hofman and Ikram16, Reference Ylikotila, Tiirikka, Moilanen, Kääriäinen, Marttila and Majamaa20 Our finding of a relatively unchanged incidence for young-onset parkinsonism suggests that genetic forms may be less prone to change over time.Reference Marras, Lang and van de Warrenburg10, Reference Schiesling, Kieper, Seidel and Kruger11 When stratified by sex, our findings are similar to previous studies that reported a more considerable decline in parkinsonism incidence rates among women over time compared to that among men.Reference Liu, Wu, Lin, Liu, Chang and Lin17, Reference Ylikotila, Tiirikka, Moilanen, Kääriäinen, Marttila and Majamaa20
Our study used a previously validated algorithm to identify patients with parkinsonism.Reference Butt, Tu and Young32 This previous study used a reference standard drawn from a primary care population, which had a proportion of Parkinson’s disease to parkinsonism of 82.7%.Reference Butt, Tu and Young32 In the UK, a previous study reported decreasing incidence of Parkinson’s disease over time, but not parkinsonism.Reference Rocca, Bower, McDonnell, Peterson and Maraganore14 Despite the trends in declining incidence found in our study, we were unable to determine whether similar trends were found for parkinsonism versus Parkinson’s disease.
For prevalence, our results add further weight to a handful of previous studies suggesting an increasing trend in prevalence.Reference Lix, Hobson, Azimaee, Leslie, Burchill and Hobson13, Reference Darweesh, Koudstaal, Stricker, Hofman and Ikram16, Reference Goldacre, Duncan, Griffith and Turner21, Reference Butt, Tu and Young32 This coincided with a decreasing trend in mortality (by 5.5%) among individuals with parkinsonism found in our study and several previous studies.Reference Guttman, Slaughter, Theriault, DeBoer and Naylor22–Reference Griffiths and Rooney24 In contrast, the standardized mortality rate of the Ontario population from 2000 to 2012 declined by approximately 19% (compared to 5.5% among persons with parkinsonism in Ontario).42 The ever increasing prevalence underscores the need to focus public health services on improving prognosis, advancing pharmacological and nonpharmacological treatment options, as well as appropriately allocating resources and facilities for patients living with parkinsonism.
Our observation of a declining incidence of mid/late-onset parkinsonism presents an opportunity to investigate drivers of the time trend and guide primary prevention strategies targeting risk factor control. We found that adjusting for known risk factors for brain health did not appreciably account for the declining incidence trends. This decline in incidence is similar to other reported declines in neurodegenerative pathologies, such as Alzheimer’s disease, that have been noted in affluent countries, and may reflect improved general health among other possible factors.Reference Jaakkimainen, Bronskill and Tierney25, Reference Langa44–Reference Langa, Larson and Crimmins46
Our study has several strengths. First, it involved a large cohort (73,129 incident cases from a source population of 10.5 million in 2013) over nearly two decades. Ontario is the most populous and ethnically diverse province in Canada, with more than 200 ethnic origins represented.42 Second, we compared trends between young-onset and mid/late-onset parkinsonism.8, Reference Grimes, Gordon and Snelgrove9 Previous literature suggests that there are etiologic and prognostic differences related to the timing of disease onset.8, Reference Grimes, Gordon and Snelgrove9 Third, we examined the prevalence of selected pre-existing conditions among persons with parkinsonism, which allows us to gain important insights about potential risk factors. Finally, we used population-based health administrative databases with a validated algorithm to ascertain parkinsonism cases.Reference Butt, Tu and Young32 This algorithm has a relatively high accuracy.Reference Butt, Tu and Young32 Most risk factors were also identified using validated algorithms with high sensitivity and specificity.Reference Chen, Kwong and Copes27–Reference Gershon, Wang, Guan, Vasilevska-Ristovska, Cicutto and To29, Reference Schultz, Rothwell, Chen and Tu31
Our study has some limitations. Using health administrative databases may not capture individuals without clinically recognized symptoms and those who have not yet sought health care. As well, the less than 100% sensitivity of the algorithm for parkinsonism may have underestimated its incidence and prevalence. In addition, despite using the entire Ontario population, this study may still have been underpowered to detect significant trends in young-onset parkinsonism (∼1% of incident cases). Nevertheless, stratified estimates of trends for the two groups can have clinical implications for etiology and prognosis of this important disease.8, Reference Grimes, Gordon and Snelgrove9 Although we know the proportion (82.7%) of patients with parkinsonism to Parkinson’s disease in the reference standard for the validation of the administrative data algorithmReference Butt, Tu and Young32 used in this study, we are unable to precisely identify the proportion in Ontario using administrative data. This is because the OHIP data only has one billing code that includes both Parkinson’s disease and parkinsonism. However, while parkinsonism encompasses different subtypes, Parkinson’s disease makes up the majority of cases (up to 80%).Reference Schrag, Ben-Shlomo and Quinn47, 48 Nonetheless, we were unable to distinguish between subtypes of parkinsonism, and some risk factors may differ among the subtypes. Finally, we were unable to ascertain other accepted or suspected risk factors (e.g., family history, drug exposure, depressive symptoms, and sleep complaints) for parkinsonism.Reference Breckenridge, Berry, Chang, Sielken and Mandel33, Reference Ascherio and Schwarzschild49–Reference Buchman, Leurgans and Yu51 Future research is needed to examine the effects of other risk factors on time trends in the incidence of parkinsonism.
Acknowledgements
This study was supported by Public Health Ontario (PHO) and ICES, which are funded by annual grants from the Ontario Ministry of Health and Long-Term Care (MOHLTC). Parts of this material are based on data and information compiled and provided by Canadian Institute for Health Information (CIHI). The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by PHO, ICES, Ontario MOHLTC, or CIHI is intended or should be inferred.
Funding
Funding for this study is provided by Health Canada (MOA-4500314182). The funding agency was not involved in the study design, analysis or interpretation of data, in the writing of the report, or in the decision to submit the paper for publication. JJW is supported by tuition assistance from the Canadian Memorial Chiropractic College. JCK, KT, and DAB are supported by Investigator Awards from the Department of Family and Community Medicine, University of Toronto. JCK is also supported by a New Investigator Award from the Canadian Institutes of Health Research.
Authors’ Contributions
JJW, HC, JCK, KT, and DAB contributed to the study design. JJW, HC, ASW, and AK prepared and cleaned the data. JJW, HC, JCK, KT, DAB, ASW, and AK contributed to the data analyses. JJW took the lead in drafting the manuscript. All authors contributed to interpretation of data, provided critical revisions to the manuscript, and approved the final draft.
Disclosures
JJW, JCK, KT, DAB, RC, ASW, BJM, AK, and HC report no disclosures.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2018.387.