Introduction
Guillain-Barré syndrome (GBS) is an autoimmune disease of the peripheral nerves and their roots, which manifests as an acute muscle weakness that usually progresses within 4 weeks.Reference Kuwabara 1 - Reference Wim Ang, Jacobs and Laman 3 Common features of this disease are monophasic course and diverse clinical outcome.
Guillain-Barré syndrome is a rapidly progressive disorder that commonly causes significant functional disability in the acute phase. Literature data, including the study conducted in our population of patients, point to the fact that more than one-third of GBS patients at nadir, as well as a quarter of patients on discharge, are chair-bound or confined to bed.Reference Peric, Milosevic and Berisavac 4 Although GBS has a favorable outcome in the majority of patients, a certain number of them could still meet poor outcome with a considerable long-term functional disability that could negatively affect their quality of life.Reference Bernsen, de Jager, van der Meché and Suurmeijer 5 - Reference Merkies, Schmitz, Samijn, van der Meché and van Doorn 7
The aim of this prospective study was to estimate the clinical outcome in GBS patients after 1 and 3 years from the acute episode of illness.
Methods
The study included GBS patients who were hospitalized in seven tertiary healthcare centers in Serbia, Republic of Srpska (Bosnia and Herzegovina), and Montenegro during 2014. The diagnosis of GBS was made according to the Brighton diagnostic criteria.Reference Sejvar, Kohl and Gidudu 8 Complete neurological evaluation was conducted exactly 1 year (±7 days) after the onset of GBS. Second retest was done in September 2017—that is, 33-44 months from acute GBS episode (median 37 months, ~3 years). This study was approved by the Ethical Board of the Neurology Clinic, Clinical Center of Serbia, and all patients signed informed consent.
Precise diagnosis of GBS and of common subtypes (acute inflammatory demyelinating polyneuropathy, acute motor axonal neuropathy, acute motor and sensory axonal neuropathy, and Miller-Fisher syndrome) was made through clinical and electrophysiological assessment.Reference Sejvar, Kohl and Gidudu 8 - Reference Dimachkie and Barohn 10 Preceding events were considered important if they occurred 3-42 days before GBS onset.Reference Sejvar, Kohl and Gidudu 8 Level of functional disability was established according to the GBS disability scale (GDS): 0, absence of symptoms; 1, patients with mild symptoms, who can run; 2, patients who can walk independently at least 5 m, but cannot run; 3, patients who can walk for 5 m or more only with assistance; 4, wheelchair-bound or confined to bed; 5, requirement for assisted ventilation; and 6, lethal outcome.Reference Hughes, Newsom-Davis, Perkin and Pierce 11 Functional disability assessment was carried out on admission, at nadir, and on discharge from the medical records, whereas assessment 1 and 3 years after acute GBS episode was carried out by local neurologists who are co-authors of the paper. Mild disability at admission, on nadir, and on discharge was considered if GDS was 0-3, and severe if it was 4-5. Poor functional outcome after 1 or 3 years was defined if GDS score was >1, whereas a score of 0-1 was considered as a favorable outcome.
One and three years after the onset of symptoms of GBS, patients were evaluated for subjective sensory deficits (paresthesia and dysesthesia), musculoskeletal pain, and fatigue. Fatigue severity was evaluated according to the Krupp’s Fatigue Severity Scale (FSS). It is a nine-item questionnaire that enables patients to assess their current condition using scores on a seven-point scale, with a score above 36 meaning significant fatigue.Reference Krupp, LaRocca, Muir-Nash and Steinberg 12 Symptom intensity of sensory deficit and musculoskeletal pain was assessed by self-assessment visual analog scale (0-10 points). Scale values 1-3 accounted for the presence of mild disturbances, 4-6 indicated moderate disturbances, and 7-10 indicated severe disturbances.Reference Peric, Peric and Rapajic 13
Normality of data was tested by the Kolmogorov-Smirnov test. Mean, standard deviation, and proportion were used as methods of descriptive statistics. χ 2 test was used to analyze the association of sociodemographic (gender, and age above or below 55) and clinical parameters (mild/severe disability at admission, nadir, and on discharge) with the disease outcome at year 1 and year 3 (including good and poor recovery estimated by GDS score, presence of sensory disturbances, musculoskeletal pain, or fatigue). We also analyzed influence of investigated parameters at year 1 on outcome at year 3 using the χ 2 test. Multivariant analysis was not performed owing to the small number of cases. Association between disability measured by GDS score, presence of sensory disturbances, musculoskeletal pain, and fatigue at year 1 and year 3 was analyzed using two hierarchical cluster analyses. In this way, we avoided multiple comparisons in a not-so-large cohort. For all statistical tests, significant testing was two-sided, with α set at 0.05 for statistical significance and 0.01 for high statistical significance.
Results
During 2014, the diagnosis of GBS was confirmed in 82 patients (Figure 1). Three patients died immediately during the hospital stay owing to sudden cardiac death, sepsis, and pulmonary embolism, and other four patients died within the next 12 months owing to sudden cardiac death (two), pulmonary embolism, and car accident. During a 1-year period, 16 patients were lost to follow-up, one patient refused to submit to further testing, whereas one patient was subsequently diagnosed with acute-onset chronic inflammatory demyelinating polyneuropathy. The remaining 57 (70%) patients underwent a complete neurological evaluation 1 year after the onset of the disease. After 3 years from the acute GBS episode, five additional patients were lost to follow-up and one died owing to malignancy. However, three patients who were lost at year 1 were retested at year 3. Thus, the final number of patients tested at year 3 was 54 (66%).
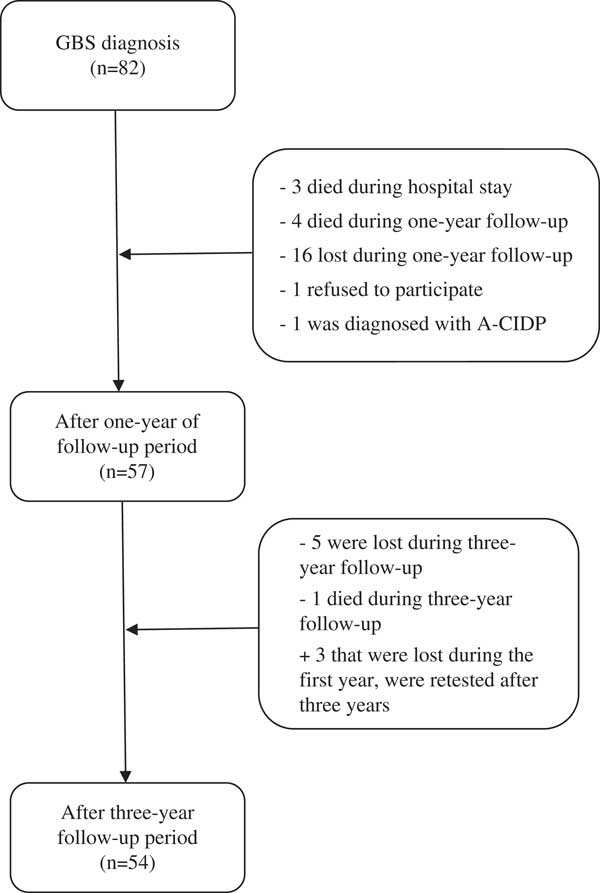
Figure 1 Flowchart representing included and excluded patients. A-CIDP=acute-onset chronic inflammatory demyelinating polyneuropathy; GBS=Guillain-Barré syndrome
Groups of subjects tested 1 and 3 years after acute phase of GBS were not different between each other and compared with the whole group of 82 patients in terms of neither sociodemographic nor clinical parameters (Table 1).
Table 1 Sociodemographic and clinical features of Guillain-Barré syndrome (GBS) patients at time of hospitalization
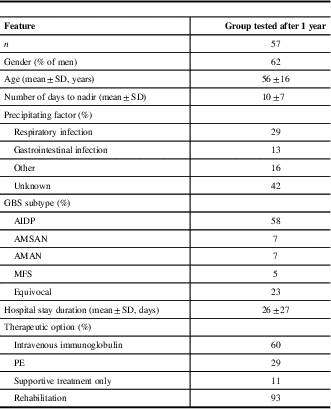
AIDP=acute inflammatory demyelinating polyradiculoneuropathy; AMAN=acute motor axonal neuropathy; AMSAN=acute motor and sensory axonal neuropathy; GDS=GBS disability scale; MFS=Miller-Fisher syndrome; PE=plasma exchange.
Poor functional outcome (GDS>1) was found in 39% of GBS patients at year 1 and 30% at year 3 (Figure 2). The presence of sensory disturbances (paresthesias/dysesthesias) was detected in 60% of our patients after 1 year and 43% after 3 years, preferably in feet (Table 2). Musculoskeletal pain was present in 40% of patients at year 1 and 33% at year 3, and it most commonly presented in the legs. Further on, significant fatigue after 1 year from acute GBS episode was found in 21% of subjects and after 3 years in only 7%.
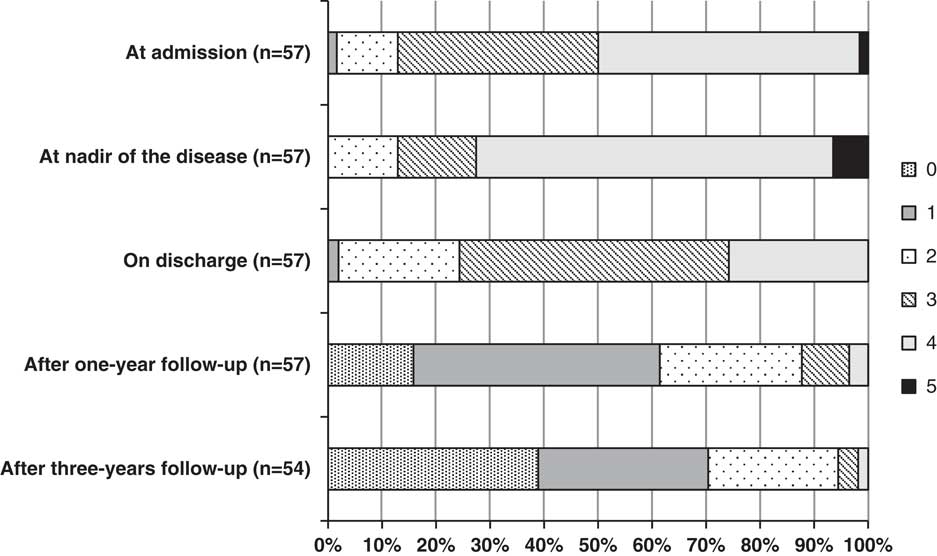
Figure 2 Functional disability in Guillain-Barré syndrome (GBS) patients at admission, at nadir, on discharge, after 1-year follow-up, and after 3-year follow-up according to GDS. GDS=GBS disability scale.
Table 2 Symptoms in Guillain-Barré syndrome patients after 1- and 3-year follow-up
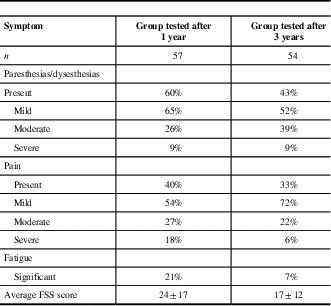
FSS=fatigue severity scale.
Cluster analysis at year 1 showed grouping in two clusters. In cluster 1 (16 patients with more severe findings), poor functional recovery was observed in 69% of patients, sensory disturbances in 88%, musculoskeletal pain in 100%, and fatigue in 56% versus 29% of patients with poor recovery, 46% with sensory symptoms, 14% with pain, and 3% with fatigue in cluster 2. Cluster analysis at year 3 showed similar results. In cluster 1 (13 patients with more severe findings), poor recovery was observed in 62% of patients, sensory disturbances in 100%, musculoskeletal pain in 100%, and fatigue in 23% and in cluster 2 poor recovery was observed in 19% of patients, sensory symptoms in 24%, pain in 12%, and fatigue in 2%.
Parameters that were proved to be associated with poor functional outcome (GDS>1) after a 1-year follow-up in our study were age >55 years at admission (p=0.05) and severe disability of the disease at admission (p<0.05) and on discharge (p<0.01) (Table 3). Poor functional outcome after 3 years was associated with male gender (p<0.05) and severe disability on discharge (p=0.06). It is also noteworthy that some clinical features observed 1 year after follow-up can be predictive of worse outcome at year 3—3.3% of GBS patients with mild disability at year 1 versus 71.4% of patients with severe disability at year 1 had severe disability at year 3 (p<0.01); also, 16.7% of patients without musculoskeletal pain at year 1 versus 52.4% of patients with musculoskeletal pain at year 1 had severe disability at year 3 (p<0.01). The presence of sensory disturbances, musculoskeletal pain, and fatigue after 1 and 3 years was not associated with any of the investigated sociodemographic and clinical parameters.
Table 3 Association between sociodemographic/clinical features and poor outcome (GDS>1) in Guillain-Barré syndrome (GBS) patients after 1 and 3 years of follow-up
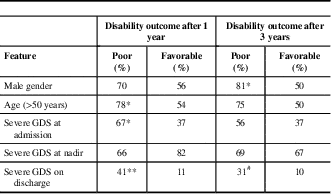
GDS=GBS disability scale.*p<0.05**p<0.01# p=0.06
discussion
Although the current study confirmed that a majority of GBS patients had a benign course of the disease with a favorable outcome, a certain number of subjects still had a significant functional disability after 1 and even after 3 years from acute GBS episode.
In our cohort of 82 patients, three patients died during the hospital stay and four died during a 1-year follow-up period (overall mortality rate of 8.5%). These findings are compatible with the literature data reporting lethal outcome in 4%-13% of GBS patients within the first year of follow-up, mostly during the acute progressive phase, and usually caused by cardiologic and respiratory complications.Reference Rees, Thompson, Smeeton and Hughes 14 - Reference Winer, Hughes and Osmond 17
After the 1-year follow-up period, 61% of patients had no symptoms or had minor symptoms of GBS, 26% were able to walk independently but unable to run, 9% were able to walk only with assistance, and 4% were bedridden or chair bound. Our results are consistent with the current literature reporting that 9%-16% of patients were unable to walk without assistance 1 year after symptom onset.Reference Rees, Thompson, Smeeton and Hughes 14 , Reference van den Berg, Bunschoten, van Doorn and Jacobs 15 , Reference Kaida 18 After the 3-year follow-up period, 70% of patients had no symptoms or had minor symptoms of GBS, 24% were able to walk independently but unable to run, 4% were able to walk only with assistance, and 2% were still bedridden or chair bound. Although there are no many studies that followed up GBS patients for 3 or more years, most of them reported favorable recovery in 67 to 100% of patients.Reference Rees, Thompson, Smeeton and Hughes 14 , Reference Winer, Hughes and Osmond 17 , Reference Dornonville de la Cour and Jakobsen 19 - Reference Chiò, Cocito, Leone, Giordana, Mora and Mutani 21 Furthermore, one recent study on GBS in children reported that after a follow-up period of 11 years, almost all of their patients had a significant recovery (97%) and only 3% were able to walk but unable to run.Reference Roodbol, de Wit, Aarsen, Catsman-Berrevoets and Jacobs 22 However, Bersano et alReference Bersano, Carpo, Allaria, Franciotta, Citterio and Nobile-Orazio 20 showed that around one-third of GBS patients changed their jobs, habits, or social activities, indicating that the impact of GBS on patients’ life is not necessarily equal to their residual disability and needs to be carefully analyzed.
Poor functional outcome of GBS after 1 year from acute episode was associated with older age (>55 years), more severe disability at admission and on discharge (i.e., slower recovery in acute phase), whereas poor functional outcome after 3 years was observed in males and in those with slower recovery in acute phase. We also observed that patients with disability and musculoskeletal pain at year 1 from acute episode of GBS had more chance to have disability at year 3. Previous studies have also analyzed the impact of various factors (age, gender, disease severity, hospital stay duration, duration of intensive care stay, the necessity for artificial ventilation, disease plateau duration) on clinical course and outcome of GBS. The most significant predictors of worse outcome after a wide range of 7 months to more than 7 years after GBS were older age, female gender, greater disability at admission, short interval between symptom onset and admission, and longer duration of plateau phase.Reference Rajabally and Uncini 16 , Reference Winer, Hughes and Osmond 17 , Reference Bersano, Carpo, Allaria, Franciotta, Citterio and Nobile-Orazio 20 , Reference Khan, Pallant, Ng and Bhasker 23 , Reference Koeppen, Kraywinkel and Wessendorf 24 Knowing the predictors of the progression and outcome of the disease allows clinicians to identify patients with potentially poor prognosis. This implies early administration of specific immunotherapy, as well as longer rehabilitation time and more frequent neurological follow-ups in order to reach a favorable outcome.
In our cohort, 60% of patients reported the presence of sensory symptoms (paresthesias/dysesthesias) one year after GBS episode and 43% after 3 years, with a majority of them having mild symptoms. Lower limbs were more often affected than upper limbs. Residual sensory symptoms were also reported in several studies in 31%-75% of patients 7 months to 11 years after the onset of GBS.Reference Roodbol, de Wit, Aarsen, Catsman-Berrevoets and Jacobs 22 , Reference Koeppen, Kraywinkel and Wessendorf 24 - Reference Bernsen, Jager, Schmitz and van der Meché 26 Most of the patients had mild lower limb paresthesias, which is consistent with our findings.Reference Bernsen, de Jager, van der Meché and Suurmeijer 5 , Reference Koeppen, Kraywinkel and Wessendorf 24 - Reference Bernsen, Jager, Schmitz and van der Meché 26
Musculoskelatal pain was present in 40% of investigated patients after 1 year of follow-up and in 33% after 3 years. A majority of patients had mild average pain—54% after 1 year and 72% after 3 years. Similar results were found in former studies, which registered mostly musculoskeletal pain but also other types of pain (neuropathic, painful paresthesias, meningism) in 33%-38% of patients, 1-2 years after disease onset.Reference Farmakidis, Inan, Milstein and Herskovitz 27 - Reference Forsberg, Press, Einarsson, de Pedro-Cuesta and Widén Holmqvist 29 Bernsen et alReference Bernsen, Jager, Schmitz and van der Meché 26 reported a higher number, 48%, of patients who had pain from 3 to 6 years after GBS onset, most prominently in the form of muscle aches. Although most of our patients reported minor pain, pain could be a permanent health problem and might highly affect functionality and quality of life of the patients.Reference Farmakidis, Inan, Milstein and Herskovitz 27 , Reference Mullings, Alleva and Hudgkins 30 Pain as a residue of GBS can frequently be neuropathic, which is of importance in differential diagnosis. Therefore, it is very important to recognize and differentiate neuropathic and musculoskeletal pain in GBS, which directs clinicians toward proper cure. Considering poorly understood and potentially multifactorial etiology of different pain types in GBS patients,Reference Farmakidis, Inan, Milstein and Herskovitz 27 it is necessary to conduct further studies to analyze the impact of various factors that could contribute to the presence and endurance of pain during the acute phase of the disease, as well as during a recovery period.
Our study revealed that one out of five patients of our cohort reported significant fatigue 1 year after symptom onset, whereas 7% of them had fatigue after 3 years. Many studies so far have shown that fatigue is one of the most disabling symptoms in GBS patients and that it could seriously affect both their functionality and quality of life.Reference Merkies and Kieseier 31 - Reference Drory, Bronipolsky, Bluvshtein, Catz and Korczyn 33 Further on, after 1 to 20 years of follow-up period, fatigue was detected in 22%-42% of GBS patients,Reference Roodbol, de Wit, Aarsen, Catsman-Berrevoets and Jacobs 22 , Reference Witsch, Galldiks and Bender 34 , Reference Rekand, Gramstad and Vedeler 35 confirming its status as one of the most important post-GBS sequela. Moreover, Drory et alReference Drory, Bronipolsky, Bluvshtein, Catz and Korczyn 33 showed that fatigue can persist and remain severe in a significant number of patients for many years after onset, in spite of making a good neurological recovery. Although etiology of fatigue remains elusive, there are some presumptions about the role of post-traumatic stress disorder as a consequence of severe neurological disease with a potentially lethal outcome. However, Rekand et alReference Rekand, Gramstad and Vedeler 35 have found that the risk of occurrence of fatigue is twice as higher in patients with residual weakness, partially explained by muscle dysfunction. The presence of fatigue can substantially affect quality of life in patients with GBS and it could potentially be treated with medications, physical therapy, and cognitive-behavioral therapy.Reference Merkies and Kieseier 31 , Reference Khan and Amatya 36 - Reference Cesarini, Angelucci, Foglietta and Vernia 39 Therefore, it is of great importance to evaluate fatigue in early stages and treat them timely with an appropriate therapy.
The main limitation of our study is a relatively small sample size that limited the number of statistical comparisons owing to possible false positive results. We are eagerly waiting data from the multi-centric IGOS study on more than 1000 patients. Another limitation is lack of data on sensory symptoms, musculoskeletal pain, and fatigue at the time of hospitalization. Finally, we had a relatively large number of patients lost to follow-up that can cause selection bias toward more severe cases that needed further medical assistance and thus came to check-up visits. On the other hand, it is also possible that some patients who were lost to follow-up actually passed away.
In conclusion, although GBS typically has a monophasic course and good outcome, a certain number of patients can have significant residual functional disability. After 1/3 years from GBS acute episode, 26%/24% of patients were unable to run, 9%/4% walked only with assistance, and 4%/2% were bedridden or chair bound. The presence of sensory symptoms (paresthesias/dysesthesias) was detected in 60% of subjects after 1 year and in 43% after 3 years, whereas pain was present in 40%/33% of patients and fatigue in 21%/7%. All these disturbances may significantly affect patients’ quality of life.
Acknowledgments
This study was supported by the Ministry of Education, Science and Technological Development of Serbia (grant #175083).
Disclosures
The authors do not have anything to disclose.







