Traumatic brain injury (TBI) is associated with 50,000 deaths and nearly 300,000 hospitalizations annually in the United States,Reference Kim, Holena, Schuster, Sims, Levine and Pascual 1 and is the worldwide leading cause of mortality in patients between the ages of 25 and 44.Reference Schaible and Thal 2 There is also a significant social cost associated with brain injury because more than 5 million Americans live with long-term neurological sequelae stemming from TBI.Reference Saiki 3
Patients with TBI are at an increased risk of venous thromboembolism (VTE), defined as either deep vein thrombosis (DVT) or pulmonary embolism (PE). Studies have reported a baseline VTE risk of approximately 5% in all hospitalized patientsReference Spyropoulos, Hussein, Lin and Battleman 4 ; however, for TBI patients, the risk of DVT is approximately 30% to 60%.Reference Schaible and Thal 2 , Reference Ekeh, Dominguez, Markert and McCarthy 5 - Reference Foreman, Schmalz and Griessenauer 12 DVT can pose significant danger to patients because roughly 20% to 30% of calf DVTs will extent proximally to the thigh if left untreated, of which approximately 40% will result in PE.Reference Praeger, Westbrook and Nichol 13 Overall, PEs occur in 1% to 24% of trauma patients.Reference Schaible and Thal 2 , Reference Geerts, Code, Jay, Chen and Szalai 7 , Reference Cherry, Nichols, Snavely, David and Lynch 14 , Reference Velmahos, Kern, Chan, Oder, Murray and Shekelle 15 Furthermore, DVTs may cause postthrombotic syndrome and chronic venous stasis, which can significantly affect patients’ quality of life.Reference Phelan 8 The exact etiology of the more than fourfold increased rate of DVTs in TBI patients is not entirely clear Reference Reiff, Haricharan, Bullington, Griffin, McGwin and Rue 11 ; however it is thought that TBIs exert a dampening effect on the physiologic dynamics of fibrinolysis, which, when coupled with the immobility caused by prolonged hospitalization creates a highly pro-thrombotic state.Reference Lu, Mahmood and Goussev 16 - Reference Tang and Lobel 18
Pharmacological venous thromboprophylaxis (VTEp) is effective in reducing rates of VTE Reference Schaible and Thal 2 , Reference Jamjoom and Jamjoom 17 ; however, some literature suggests that VTEp increases the risk of intracranial hemorrhage (ICH) progression Reference Levy, Salottolo and Bar-Or 19 , Reference Haddad and Arabi 20 ; therefore, many physicians are hesitant to administer VTEp for fear of causing secondary progression of TBI.Reference Jamjoom and Jamjoom 17 Unfortunately, this delay may cause undue risk from VTE. Clinical decisions must therefore balance the risk of VTE with the potential to cause iatrogenic ICH progression.
There is currently insufficient research to produce formal clinical guidelines on optimal timing of VTEp.Reference Gross, Norman and Cook 9 , Reference Depew, Hu, Nguyen and Driessen 21 In 2002, the Eastern Association for the Surgery of Trauma (EAST) recommended that the safety of VTEp, both low-molecular-weight heparin (LMWH) and unfractionated heparin (UFH) in TBI was not established and VTEp administration should be tailored to each patient.Reference Rogers, Cipolle, Velmahos, Rzycki and Luchette 22 Similarly, both the American College of Chest Physicians (ACCP) and Brain Trauma Foundation (BTF) recommend VTEp in lieu of mechanical prophylaxis as soon as possible after sustaining a TBI, but could not recommend a specific time frame within which VTEp can be safely administered.Reference Geerts, Bergqvist and Pineo 23 , Reference Carney, Totten and O’Reilly 24
Clinical decision tools for VTE prevention improve VTEp adherence, and the ACCP guidelines recommend formal VTE prevention protocols for hospitalized patients.Reference Geerts, Bergqvist and Pineo 23 With this in mind, we set out to review the literature to determine the risk of TBI expansion after VTEp with the ultimate goal of determining how long clinicians should wait after TBI before administering VTEp. A secondary objective of this research was to lay the foundation for a clinical practice guideline (CPG) directing timing of VTEp therapy in TBI patients.
Methods
The primary objective of this study is to determine the safety of administering VTEp (LMWH or UFH) to TBI patients, and in particular, assessing if VTEp causes intracranial hemorrhagic expansion. The outcome of interest is whether VTEp causes hemorrhagic progression in TBI patients, and if so, within what time frame postinjury. The population of interest includes all hospitalized patients with TBI who were not on anticoagulation preinjury or known to have a preexisting coagulopathy. This systematic review was reported in accordance with the Preferred Reporting of Items for Systematic Reviews and Meta-Analyses protocols.Reference Moher, Shamseer and Clarke 25 The literature was searched using databases MEDLINE and EMBASE. We limited our findings to English language studies published between January 1, 1999, and December 1, 2015. A combination or partial combinations of the following search terms were used: traumatic brain injury, venous thromboembolism, anticoagulant agent, anticoagulants, fibrinolytic agent, prophylaxis, thromboprophylaxis, chemoprophylaxis, brain injuries, and venous thrombosis. (Please see the supplementary appendix for detailed search strategy.) Animal studies, articles about patients taking anticoagulants before TBI and articles on nontraumatic intracranial hemorrhage (hemorrhagic stroke) were excluded. Articles with short titles such a “Discussion” or “Author Reply” and those without an abstract were excluded. Titles of articles were first reviewed for relevance. Abstracts of selected articles were then reviewed for inclusion in the study. Two independent investigators (JM and CD) then evaluated full texts of selected articles. Reference sections of the articles were reviewed to identify additional relevant studies.
Two investigators (JM and CD) independently extracted data from the included studies and assessed the methodological quality based on the Oxford Centre for Evidence Based Medicine Levels of Evidence.Reference Phillips, Ball, Sackett, Badenoch and Straus 26 Each study was given an evidence class score ranging from 1a (high-quality randomized controlled trials [RCTs]) to 4 (case series and poor-quality case-cohort studies). A score of 1b was given to RCTs with narrow confidence intervals, 2a to systematic reviews with homogeneity, 2b to low-quality RCTs and retrospective cohort studies, 3a to systematic reviews with heterogeneity or case control studies, and 3b to individual case control studies. Data were extracted from the included studies using a standardized form. We collected authors, date of publication, journal of publication, a descriptive evaluation of each study, research design, timing of VTEp, study limitations, and main findings (Table 1). RCTs were assessed for bias based on the Cochrane Collaboration ToolReference Higgins, Altman and Gotzsche 27 and cohort studies were evaluated for bias using the Newcastle-Ottawa Scale.Reference Wells, Shea, O’Connell and Robertson 28
Table 1 Systematic review of 21 sudies

AIS=Abbreviated Injury Score; ASA=aspirin; BID=2 times daily; CI=confidence interval; IPC=intermittent pneumatic compression; ISS=Injury Severity Score; IV=intravenous; OR=odds ratio; RR=risk ratio; SC=subcutaneously; TID=3 times daily.
* Twice a day dosing.
† Dosing every 8 hours.
‡ Intermittent pneumatic compression.
Statistical analyses were performed using Stata 12.1 software package (College Station, TX). Pooled rate estimates from the studies were plotted against VTEp timing (i.e. no VTEp vs VTEp given within 24 hours of injury, 48 hours of injury, 72 hours of injury, and >72 hours after injury). We performed univariate meta-regression analysis in an attempt to identify a relationship between VTEp timing and hemorrhagic progression and assess study heterogeneity (variance between studies) using an I 2 statistic.
After the formal literature review, a consultation process with two trauma surgeons, two intensivists, and a neurosurgeon was carried out to review the literature findings. The consultation process occurred over several meetings and involved reviewing all available literature. Expert opinion, based on years of clinical work in trauma, critical care, and neurosurgery, was used to supplement the data from the review and to attain consensus opinion on a CPG.
Results
Eighty-one articles from the MEDLINE database and 332 from the EMBASE database were selected for abstract review (Figure 1). Forty-five articles were reviewed in entirety, of which 21 were included in the systematic review. Reasons for exclusion after full review were: no data regarding VTEpReference Algattas and Huang 29 , Reference Cowley and da Silva 30 data from rehabilitation centers,Reference Carlile, Nicewander, Yablon, Brown, Brunner, Burke, Chae, Englander, Flanagan and Hamond 31 , Reference Elliott, Patel, Matharu, Amos, Machin, Liu and Greenwood 32 no data on TBI progression,Reference Reiff, Haricharan, Bullington, Griffin, McGwin and Rue 11 , Reference Praeger, Westbrook and Nichol 13 , Reference Haddad and Arabi 20 , Reference Depew, Hu, Nguyen and Driessen 21 , Reference Grenander, Bredbacka and Rydvall 33 , Reference Vergouwen, Roos and Kamphusien 34 investigating patients on oral anticoagulation before arriving in the hospital,Reference Schoonman, Bakker and Jellema 35 published critique of previous article,Reference Petruska 36 literature reviews of studies already captured by our review,Reference Schaible and Thal 2 , Reference Saiki 3 , Reference Phelan 8 , Reference Gross, Norman and Cook 9 , Reference Foreman, Schmalz and Griessenauer 12 , Reference Tang and Lobel 18 , Reference Phelan 37 - Reference Carlile, Yablon, Mysiw, Frol, Lo and Diaz-Arrastia 39 no data on timing of VTEp,Reference Cothren, Smith, Moore and Morgan 40 and a study investigating inferior vena cava (IVC) filters.Reference Cherry, Nichols, Snavely, David and Lynch 14
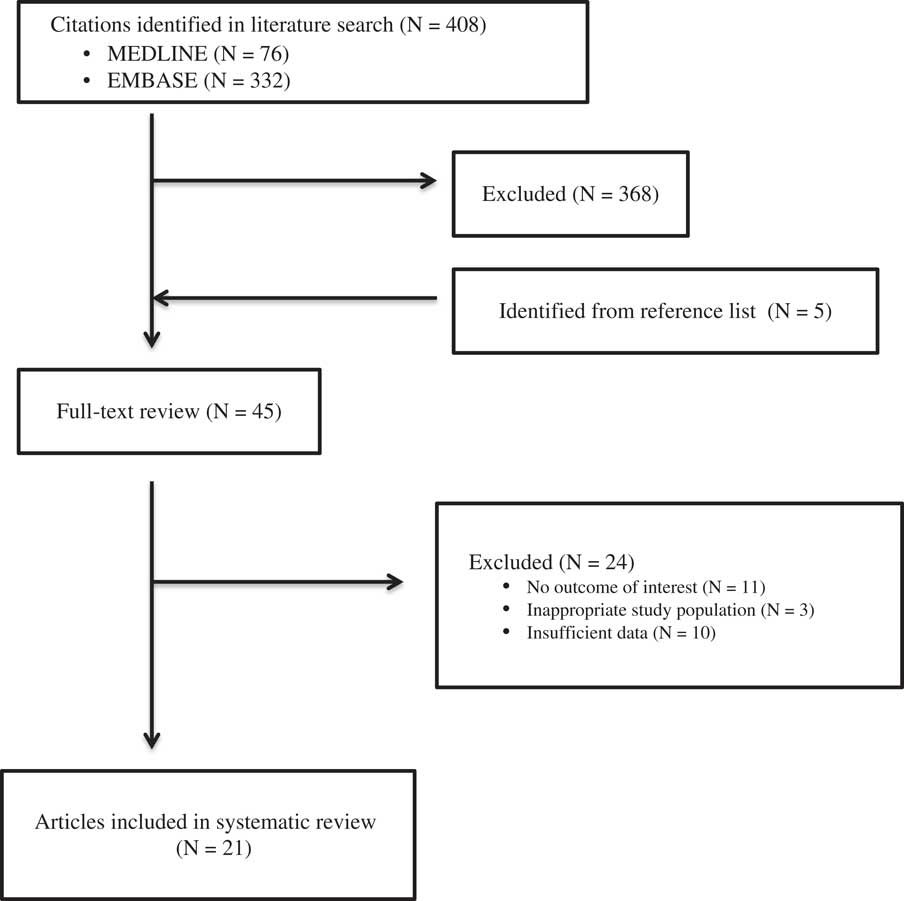
Figure 1 Methods Flow Diagram
Eighteen total studies demonstrated that VTEp postinjury in patients with stable head computed tomography (CT) scan does not lead to radiographic or clinical TBI progression. Fourteen studies demonstrated that VTEp administration specifically 24 to 72 hours postinjury is safe in patients with stable injury. Four studies suggested that administering VTEp specifically within 24 hours of injury in patients with stable TBI does not lead to progressive ICH. However, one study, a retrospective review with multivariate analysis of 1215 patients, suggested LMWH is a risk factor for TBI progression, defined as “progressive bleed on follow up CT scan,”Reference Kwiatt, Patel and Ross 41 and another retrospective review found UFH was associated with higher rates of TBI progression, defined as worsening bleeding on repeat CT head, than LMWH.Reference Minshall, Eriksson, Leon, Doben, McKinzie and Fakhry 42 A study by Lin et al divided TBIs into those with ICH and without ICH. They determined that the rate of ICH progression when VTEp was administered within 48 hours was 10.6% in those with preexisting ICH compared with 0.7% in those without ICH.Reference Lin, Davis and Wong 43
Meta-regression analysis of the studies demonstrated that there was no relationship between rate of hemorrhagic progression and VTEp timing. Overall, the timing of DVT prophylaxis only explains 1.56% of the variations between rebleeding rates reported in different studies, which is not statistically significant (p=0.31). Because of the high level of heterogeneity (variation between studies, I 2 >95%) detected by the meta-analysis, we did not report weighted-pooled summary estimates for rebleeding rate in this review. Four of 12 studies directly comparing VTE rates between early and late VTEp administration reported a significant decrease in VTE rates with early VTEp administration.Reference Jamjoom and Jamjoom 17 , Reference Kwiatt, Patel and Ross 41 , Reference Farooqui, Hiser, Barnes and Litofsky 46 , Reference Scudday, Brasel and Webb 47 Similarly, another seven studies found VTEp rates of only 0.0% and 2.0% in patients given VTEp within 24 to 72 hours of injury. Three other retrospective reviews found somewhat higher rates of VTE that ranged from 7.3% to 14%.Reference Depew, Hu, Nguyen and Driessen 21 , Reference Dudley, Aziz and Bonnici 54 , Reference Kim, Gearhart, Zurick, Zuccarello, James and Luchette 57 Summary of literature can be found in Table 1; see Table 2 for risk of bias assessment of RCTs based on the Cochrane Collaboration tool.Reference Phillips, Ball, Sackett, Badenoch and Straus 26 Only seven of the 18 nonrandomized cohort studies reviewed were deemed “low risk” for bias according to Newcastle-Ottawa Scale (Table 3).Reference Higgins, Altman and Gotzsche 27 A preliminary CPG was formulated based on data from this review and consensus opinion among clinical experts (Figure 2).
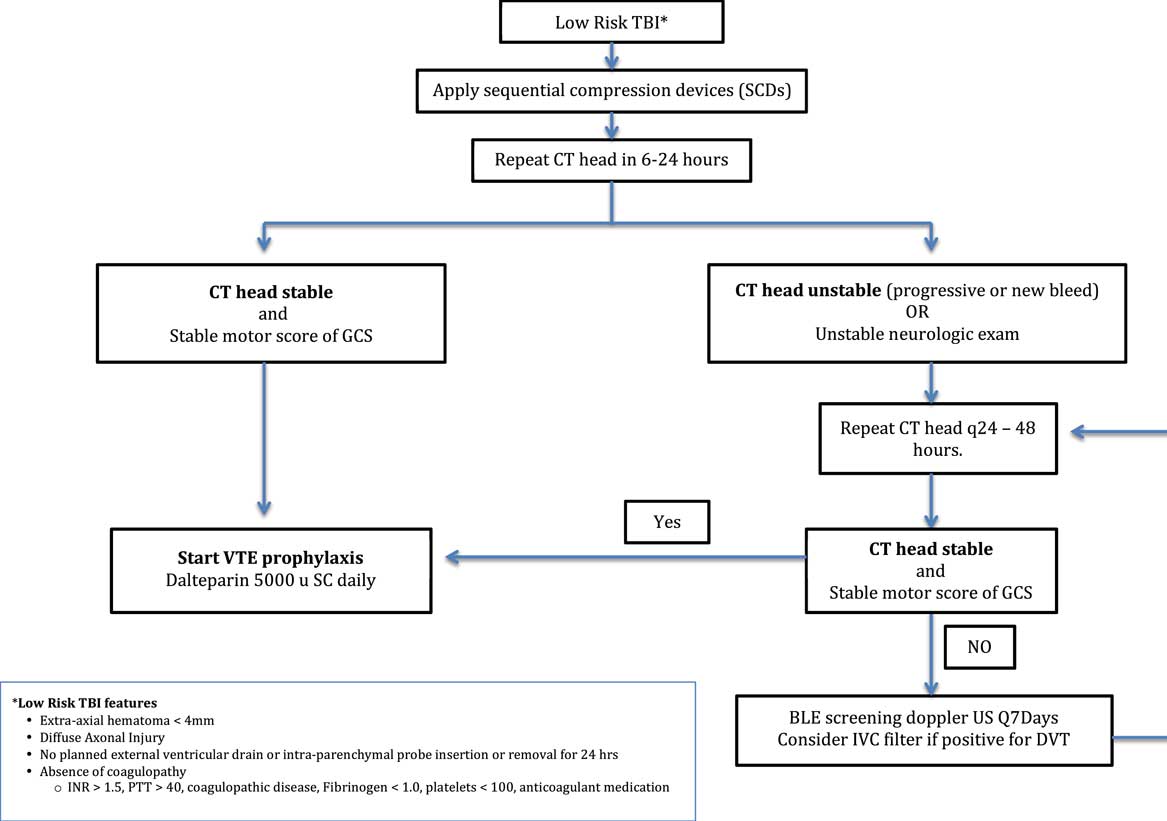
Figure 2 Clinical Practice Guidelines for Venous Thromboembolism Prophylaxis in Patients with low risk TBIs.
Table 2 Cochrane Collaboration Tool for assessing risk of bias for randomized controlled trials
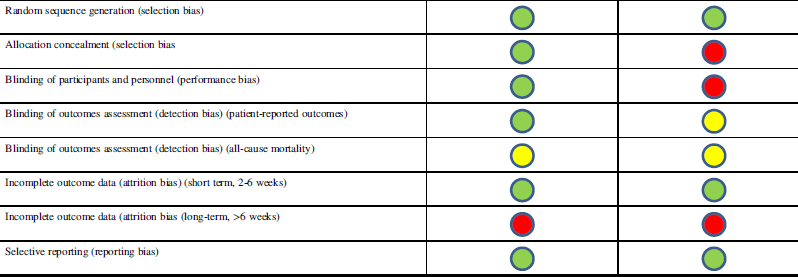
![]() Low risk of bias
Low risk of bias
![]() High risk of bias
High risk of bias
![]() Unsure risk of bias
Unsure risk of bias
Table 3 Newcastle-Ottawa Scale Assessment of bias for cohort studies
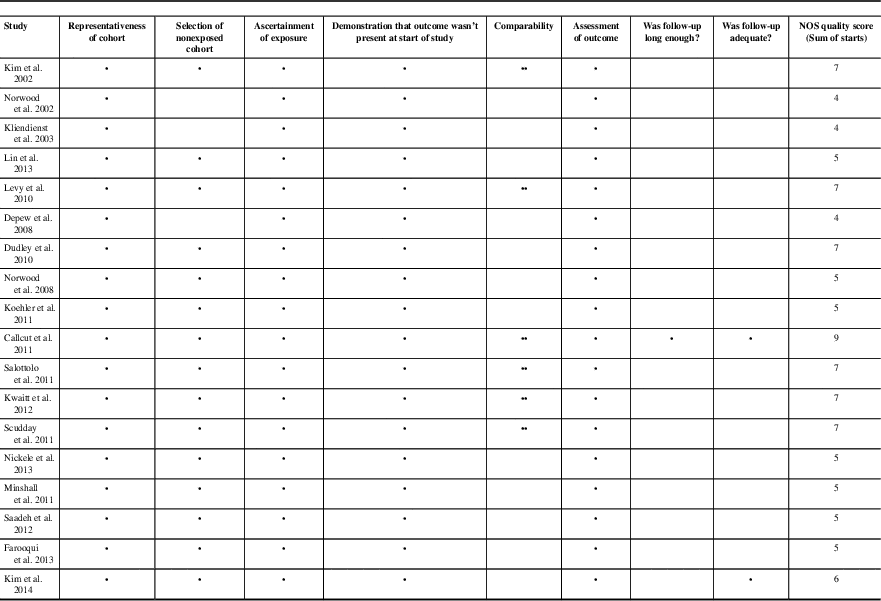
Full mark for Newcastle-Ottawa Scale was 9 points. Scores ≥7 were considered high quality.
* The corresponding paper scores one point in this category.
** The corresponding paper scores two points in this category.
Discussion
TBI is a common cause of death and disability in young patients.Reference Schaible and Thal 2 Preventing secondary brain injury is the primary objective of TBI management, yet complications of VTE can be devastating. Clinicians should be weary of iatrogenic hemorrhagic progression secondary to VTEp; however, delaying chemoprophylaxis may cause unwarranted increases in morbidity and mortality. Decisions regarding VTEp timing are largely based on individual experience because ACCP, BTF, and EAST guidelines are unable to support any recommendations regarding timing of VTEp initiation other than recommending that VTEp be initiated once hemorrhagic risk decreased.Reference Rogers, Cipolle, Velmahos, Rzycki and Luchette 22 , Reference Carney, Totten and O’Reilly 24
The efficacy of chemoprophylaxis in preventing VTE in hospitalized patients is well established.Reference Agnelli, Piovella and Buoncristiani 44 In trauma patients, delaying VTEp for 72 hours after injury doubles the risk of VTE,Reference Jamjoom and Jamjoom 17 whereas delaying VTEp 96 hours after injury increases the risk threefold.Reference Nathens, McMurray and Cuschieri 45 In our review, four studies demonstrated that early administration of VTEp is associated with decreased rates of VTE,Reference Jamjoom and Jamjoom 17 , Reference Kwiatt, Patel and Ross 41 , Reference Farooqui, Hiser, Barnes and Litofsky 46 , Reference Scudday, Brasel and Webb 47 whereas another seven studies identified VTE rates of only 0.0% and 2.0% in patients given VTEp within 24 to 72 hours of injury. This rate is much lower than commonly documented rates of VTE in TBI patients, further that early administration of VTEp reduces incidence of VTE. Three studies, however, found rates of VTE ranged from 7.3% to 14%, despite administering VTEp within 72 hours of injury.Reference Depew, Hu, Nguyen and Driessen 21 , Reference Dudley, Aziz and Bonnici 54 , Reference Kim, Gearhart, Zurick, Zuccarello, James and Luchette 57 Although rates of VTE in these three studies are relatively high despite early VTEp administration, patients in these studies were subject to routine screening with Doppler ultrasound; therefore, clinicians likely discovered subclinical DVTs that might not have been identified in other studies.
There are no consensus guidelines to recommend preferred agent, dose, or timing of chemical VTEp. The BTF guidelines suggest either UFH or LMWH because both are efficaciousReference Carney, Totten and O’Reilly 24 ; therefore, the decision of whether to use UFH or LMWH is largely based on practitioner and institution preference. LMWH has demonstrated superiority in PE prevention compared with UFH in large studies, however. For instance, a large, multicenter retrospective study on trauma patients demonstrated that LMWH was associated with nearly one-half the rate of PEs compared with UFH.Reference Byrne, Geerts and Mason 58 Additionally, LMWH is associated with decreased rates of heparin-induced thrombocytopenia.Reference Greinacher, Alban, Omer-Adam, Weitschies and Warkentin 59 As such, many trauma centers, including ours, favor LMWH as the agent of choice for chemical prophylaxis; however, UFH has a shorter half-life and is more easily reversed and thus may be the preferred agent when risk of hemorrhagic TBI progression is at stake. Clearly, there is a need for large-scale randomized trials to definitively investigate the potential benefits of LMWH compared with UFH in the setting of TBI.
Our primary objective was to determine if administering VTEp within 24 to 72 hours of TBI is safe or is dangerously associated with intracerebral hemorrhagic progression. Although the majority of evidence is retrospective, our study demonstrated that the practice of early VTEp administration after TBI is generally safe. Specifically, our review identified 18 studies that demonstrated that administering VTEp to patients with stable injury demonstrated by repeat head CT scan does not lead to progression of TBI. For example, Farooqui et alReference Farooqui, Hiser, Barnes and Litofsky 46 initiated a protocol in which all patients with TBI received either Enoxaparin or UFH 24 hours postinjury as long as patients were without coagulopathy or progressive hemorrhage seen on repeat head CT scan. Forty-eight to 72 hours after initiation of VTEp, all patients received a third CT scan of the head to assess for TBI progression. Investigators found that there were no significant differences in Glasgow Coma Scale (GCS) or hemorrhagic progression between patients who received early and late VTEp. The investigators also found that patients who received early VTEp had significantly shorter hospital stays (12.3 days vs 7.4 days, p < 0.05), shorter intensive care unit stays (9.2 days vs 5.0 days, p < 0.001), and fewer VTEs (5.6% vs 0.0%, p < 0.01) than those who were not included in the early VTEp protocol. However, the early (protocol) group had lower overall Injury Severity Scores than the nonprotocol group, thus potentially confounding these results. Similarly, Saadeh et alReference Saadeh, Gohil and Bill 10 conducted a retrospective review of 122 TBI patients who were administered VTEp with LMWH 24 to 48 hours after stable head CT. Investigators found that none of the patients who received VTEp after TBI developed hemorrhagic progression; however, results from this study must be interpreted with caution because only 49.5% of eligible patients actually received VTEp within the 24- to 48-hour window. In a larger retrospective analysis study, Scudday et alReference Scudday, Brasel and Webb 47 found that 242 of 402 TBI patients had VTEp administered 24 to 72 hours after stable head CT scans. In fact, patients administered early VTEp had significantly lower GCS and Abbreviated Injury Scale scores than those not administered VTEp. Interestingly, despite having more severe brain injury, there was no significant increase in hemorrhagic progression between patients receiving early VTEp (3% rate of progression) and patients not receiving VTEp (6% rate of progression).
The potential devastating morbidity and mortality associated with hemorrhagic TBI progression makes performing randomized prospective trials on VTEp initiation in TBI patients practically and ethically challenging; however, we did identify two prospective studies. A randomized, double-blinded, noninferiority clinical trial conducted by Phelan et alReference Koehler, Shipman, Davidson and Guillamondegui 49 demonstrated that administering VTEp within 48 hours of injury was associated with a TBI progression rate of 5.9%, which was not significantly different from placebo. The authors loosely defined TBI progression as a finding on repeat CT scans of the head of “worse” hemorrhage compared with the initial CT scan of the head. Interestingly, all radiographic TBI progressions were subclinical. The authors of this study limited their subjects to patients with TBIs deemed “low risk” for rebleeding, which were subdural hematomas or epidural hematomas smaller than 8 mm, intraventricular hemorrhage smaller than 2 cm, small contusions, and small subarachnoid hemorrhage with normal CT angiogram of the head and neck vessels. This criteria, known as the Berne-Norwood Criteria, was conceived by Norwood et al in 2008.Reference Norwood, Berne, Rowe, Villarreal and Ledlie 6 In their prospective cohort study, the authors enrolled “low-risk” TBI patients to receive VTEp within 48 hours of admission. Hemorrhagic progression was found in 18 (3.4%) patients after starting VTEp. Ten of the 18 total hemorrhagic transformations and five of the six clinically significant hemorrhagic transformations were from protocol violations. Had the protocol violations been withheld from the analysis, the rate of TBI progression after VTEp would have dropped to 1.8%. This study thus demonstrated that, in low rebleeding risk patients (as defined by the Berne-Norwood Criteria), VTEp can be administered safely within 48 hours of admission.
It is difficult to ascertain the natural history of TBIs and the baseline rate of hemorrhagic progression that occurs regardless of VTEp administration. For example, a retrospective cohort study by Kwiatt et alReference Kwiatt, Patel and Ross 41 divided TBI patients into those who had received VTEp within 48 hours of admission, 48 hours to 7 days postadmission, and more than 7 days postadmission. The authors found that radiologic TBI progression occurred in 24% of patients who were not administered VTEp and that the rates of hemorrhagic progression did not increase once VTEp was given. In fact, there was no difference in the rate of hemorrhage progression after receiving VTEp regardless of when it was initiated. Of the patients who received VTEp within 48 hours, 22% had hemorrhage progression, which was similar to the rate of hemorrhagic progression in patients who had received LMWH 7 days postinjury, suggesting that timing of VTEp has no effect on TBI progression in this cohort. In total, 9.9% of patients with a stable repeat CT scan of the head before initiation of VTEp had progression after receiving VTEp. In this series, multivariate analysis implicated VTEp as the strongest risk factor for hemorrhagic progression (odds ratio [OR], 2.41). This study included patients who required emergency neurosurgical intervention, which may explain the high baseline progression rate of 24%; however, deciphering which patients had TBI progression secondary to the natural history of TBIs regardless of LMWH is challenging.
That subtle hemorrhagic progression is frequently seen in repeat head CT scans indicates that subclinical progression is likely part of the natural history of TBIs. Furthermore, as CT scanning technology continues to improve, we are likely to continue identifying even more subtle changes in cerebral hemorrhage morphology. As such, the baseline rates of radiographic TBI progression in the absence of VTEp are variable. For example, Saddeh et alReference Saadeh, Gohil and Bill 10 reported baseline hemorrhagic progression rate of 13.9%, Norwood et al (2002)Reference Norwood, McAuley and Berne 55 found a baseline progression rate of 19%, Callcut et alReference Callcut, Hanseman and Solan 53 found baseline progression rate at 6.25%, Scudday et alReference Scudday, Brasel and Webb 47 reported a baseline progression rate of 6%, Phelan2012Reference Phelan, Wolf and Norwood 51 found a rate of 3.5%, and Norwood et al (2008)Reference Norwood, Berne, Rowe, Villarreal and Ledlie 6 determined that 8.3% of their subjects had progression at baseline. One potential explanation for the variance in baseline hemorrhagic progression is that timing of a second CT scan of the head differs between studies. It is quite plausible that a repeat CT scan of the head performed earlier is more likely to demonstrate baseline hemorrhagic progression than one that is performed later after the traumatic event. However, although the rates of baseline TBI instability vary according to the literature, it may be acceptable to deem VTEp “safe” if its administration does not cause the rate of TBI progression to rise significantly over the baseline.
Ultimately, data from our review indicate that early VTEp is safe in patients with TBI; however, most of the high hemorrhagic risk patients have been excluded from analysis. As a result, there is insufficient evidence to generalize VTEp safety in all TBI patients. We have created a preliminary CPG for use in patients with TBI and low hemorrhagic risk features (Figure 2). This CPG is based on our systematic review of the literature and expert consultations with intensivists, neurosurgeons, and trauma surgeons at a Canadian Level-1 trauma center. The CPG, which has not yet been implemented, places patients into two protocol arms: high hemorrhagic risk and low hemorrhagic risk. The CPG highlights the need for clinicians to weigh hemorrhage and thrombosis risk on a patient-by-patient basis. Early and systematic identification of those patients with low risk of delayed bleeding would allow early VTEp initiation and therefore reduce the risk of VTE in a substantial segment of the TBI population. Conversely, early classification of patients into a high hemorrhagic risk category would allow us to minimize the risk of iatrogenic TBI progression resulting from anticoagulation. Studies have shown that elevated prothrombin time and international normalized ratio, antiplatelet medications, and prior anticoagulants are risk factors for progression.Reference Allison, Nakagawa, Hayashi, Donavan and Koenig 60 - Reference Kelly, Rudd, Lewis and Hunt 62 As a result, our CPG defines high hemorrhagic risk TBIs as extra axial hematomas >4 mm, diffuse axonal injury, TBIs requiring neurosurgical intervention, and TBIs occurring in patients with an international normalized ratio >1.5, a partial thromboplastin time >40 seconds, a platelet count of <100 × 109/liter, a known coagulopathy, and patients taking oral anticoagulants and/or anti-platelets medications. Patients with low hemorrhagic risk features and stable TBI would be allocated to a treatment pathway that supports administering VTEp with dalteparin 5000 units subcutaneously within 24 hours of injury. Patients with unstable TBI and/or high hemorrhagic risk features would be shunted into a pathway that prioritizes mechanical VTEp, promotes early detection of DVT, and facilitates ongoing assessment hemorrhage risk based on serial head CTs. The BTF, EAST, and ACCP guidelines support the use of mechanical thromboprophylaxis with intermittent pneumatic compression whenever possible.Reference Rogers, Cipolle, Velmahos, Rzycki and Luchette 22 , Reference Carney, Totten and O’Reilly 24 Therefore, all patients in the high-risk arm of the CPG would be administered mechanical thromboprophylaxis. Our CPG would also recommend screening for DVTs clinically on daily rounds and every 7 days with Doppler ultrasound in patients in the high-risk arm in the hopes of more quickly identifying subclinical DVTs. Last, the EAST guidelines make Level III recommendations to consider IVC filters in trauma patients who cannot receive anticoagulationReference Rogers, Cipolle, Velmahos, Rzycki and Luchette 22 ; therefore, our CPG also recommends consideration of an IVC filter if a DVT is found. Our next objective is to implement the CPG in TBI patients admitted to the intensive care unit and trauma services and compare rates of VTEs between historical controls and prospective patients treated in accordance with the CPG.
To our knowledge, this is the most extensive review of early VTEp administration in TBI patients, but there are several limitations of this study. For example, our meta-regression analysis did not detect a statistically significant relationship between timing of VTEp and hemorrhagic progression; however, because of study heterogeneity it is not possible to tell if the timing of VTEp truly has no influence on hemorrhagic progression or if the included studies are simply too heterogeneous to detect such an association. Additionally, most of the studies in our review did not specify which type of ICH (e.g. subarachnoid hemorrhage, epidural hematomas, subdural hematomas, contusions) were at higher risks of rebleeding following VTEp. We use TBI as an umbrella term for cerebral hemorrhage; however, in reality, TBI is a heterogenous group of injuries with different clinical features that are likely to respond differently to VTEp. Future research could focus on clarifying what characteristics of TBI are higher risk for progression following VTEp administration. We are also critical of using radiologic criteria to determine TBI progression; for example, dichotomizing TBI radiographically as either having “progressed” or “not progressed” fails to quantify the degree of expansion and association with clinical sequelae. Future research could focus on correlating radiographic TBI progression with clinical neurologic status. Also, lack of DVT screening in many of the studies has likely caused us to underestimate the true rates of VTE. Research on the utility of weekly DVT screening in trauma patients who are not administered VTEp could be an important area of future study because the long-term sequelae of subclinical DVTs may include postthrombotic syndrome, venous stasis, and delayed rehabilitation.Reference Kelly, Rudd, Lewis and Hunt 62 Last, our preliminary CPG was developed based on a combination of findings identified from this review and expert opinion from a single center. Certainly, expert opinion from a single center represents the lowest grade of evidence, and the CPG itself is meant for low hemorrhagic risk patients, which limits its generalizability. Creating protocols for VTE prevention in high hemorrhagic risk TBI patients, such as those requiring neurosurgical intervention, was beyond the scope of this review, but is an important area for potential future research.
In conclusion, we have systematically reviewed the literature to determine that it is likely safe to administer VTEp 24 hours postinjury in clinically stable patients who have sustained a low hemorrhage risk TBI that has not progressed on repeat CT scan of the head. Future research is needed to identify specific risk factors for hemorrhagic progression that may preclude early VTEp administration. Until such factors are clearly delineated, we recommend a relatively conservative, yet consistent approach that includes administering VTEp within 24 hours of injury in patients with extra-axial hematomas smaller than 4 mm who are without coagulopathy or anticoagulant medications and have a radiographically and clinically stable TBI.
Acknowledgments
We would like to thank Niki Baumann, Librarian at the College of Physicians and Surgeons of British Columbia Library, for her dedication and assistance with this review.
Disclosures
The authors have nothing to disclose.
Statement of Authorship
JM: Literature search, study design, data collection, data interpretation, writing and critical revision. CD: Literature search, study design, data collection, data interpretation, critical revision. KD: Study design and critical revision. WC: Statistical analysis and critical revision. DCE: Study design and critical revision. MSS: Study design and critical revision. NG: Study design and critical revision. DEGG: Study design and critical revision. PG: Study design and critical revision. SMH: Principal investigator, study design, critical revision.
Appendix
Literature Search Strategy
Literature search was performed with the assistance of the College of Physicians and Surgeons of British Columbia (CPSBC) librarian, using the CPSBC library access to EMBASE and MEDLINE databases.
Medline (Through the PubMed Interface)
“Brain Injuries/therapy”[Mesh]
AND
“Venous Thrombosis/prevention and control”[Mesh] OR “Venous Thromboembolism/prevention and control”[Mesh] OR “Anticoagulants”[Mesh] OR “Fibrinolytic Agents”[Mesh] AND “Thrombolytic Therapy”[Mesh]
AND
english[lang] AND “Humans”[Mesh]
OR
“Brain Injuries”[Mesh]
AND
prophylaxis[tiab]
AND
“Venous Thrombosis/prevention and control”[Mesh] OR “Venous Thromboembolism/prevention and control”[Mesh] OR “Anticoagulants”[Mesh] OR “Fibrinolytic Agents”[Mesh] AND “Thrombolytic Therapy”[Mesh]
AND
english[lang] AND “Humans”[Mesh]
Limits: 1999+
EMBASE
exp *traumatic brain injury/dm, dt, rh, th [Disease Management, Drug Therapy, Rehabilitation, Therapy]
AND
exp venous thromboembolism/pc [Prevention] OR exp anticoagulant agent/ OR exp fibrinolytic agent/ OR exp fibrinolytic therapy/ OR (anticoagula* OR prophyla* OR chemoprophyla* OR thromboprophyla* OR antithromb*).ti,ab.
NOT
(book or book series or conference abstract or conference paper or conference proceedings or “conference review” or short survey or trade journal).pt.
NOT
exp animal/ not exp human/
⇒ NOT HELPFUL AT ALL!
Second try:
exp *traumatic brain injury/
AND
exp venous thromboembolism/pc [Prevention] OR exp anticoagulant agent/ OR exp fibrinolytic agent/ OR exp fibrinolytic therapy/ OR (anticoagula* OR prophyla* OR chemoprophyla* OR thromboprophyla* OR antithromb*).ti,ab.
NOT
(seizure prophylaxis OR preinjur* OR pre-injur*).ti,ab.
NOT
(book or book series or conference abstract or conference paper or conference proceedings or “conference review” or short survey or trade journal).pt.
NOT
exp animal/ not exp human/
⇒ much better!
Limits: English, 1999+, remove duplicates
Search Strategies
Some syntax that were used to describe search strategies:
AND means the article must contain at least one of the terms in the line above, and at least one of the terms below, in order to appear in the search results.
OR means the article must contain at least one of the terms in order to be found. Used for synonyms e.g. Venous Thrombosis OR Venous Thromboembolism
NOT means the article must not contain any of the search terms in the line below. e.g. NOT trade journal.pt. removes any results from trade journals from the search results.
The PubMed interface to Medline uses these tags:
[Mesh] means Medical Subject Headings. These are labels placed on each article to show what the article is about. These are highly effective, as they are “exploded” – that is, a search for “Anticoagulants”[Mesh] will search for more specific types of anticoagulants, such as Antithrombin Agents.
[tiab] means a search for this word in the title and abstract of all available articles
The EMBASE database uses different syntax:
exp something/ means that this term was searched as an Emtree Subject Heading. These are labels like Medical Subject Headings, just a different set because it’s a different database. The “exp” part stands for explode, and works the same way as in PubMed, for example, exp venous thromboembolism/ includes the search deep vein thrombosis/ automatically.
asterisk (*) between the exp and the term means that one is searching for articles where this Subject Heading is considered to be the main focus of the article – that is, most if not all of the article is about this concept. It is particularly useful in the EMBASE database to eliminate irrelevant results.
(something).ti,ab. searches for the search word in the title and abstract of all available articles
asterisk (*) means any ending for the word. E.g. anticoagula* will search for anticoagulant, anticoagulants, anticoagulation, anticoagulate, and so forth.
(something).pt. searches for particular document types. I often use this when I receive a large number of results, in order to remove items which are irrelevant (such as trade journals) and items which are hard to obtain (such as books and conference publications).
exp animal/ not exp human/ is a way of removing animal studies without removing articles about humans. Unlike Medline, EMBASE is not as consistent about labeling their Human studies, so this way removes any obviously irrelevant articles while leaving the unlabelled ones intact.
pre-injur*.ti,ab. searches for both “pre injury” and “pre-injury” automatically.
Remove duplicates is useful in the Ovid version of EMBASE (which I used), to find any articles which have been entered into the database twice, and remove the extra copies.







