Case Presentation
An 11-year-old female patient presented to our center for ventriculoperitoneal shunt check-up after a period of unexplained nausea. The patient’s family immigrated from another country 1 year before the presentation. The patient received the first shunt at the age of 1 week for aqueductal stenosis that was revised after 6 months for a shunt infection. She experienced an uneventful period until this presentation. At our facility, shunt series (X-ray) and brain MRI were performed, and shunt failure was excluded. Interestingly, her brain MRI revealed a unique cerebellar anomaly that was not reported in her previous medical documents. The patient’s developmental assessment revealed that she has learning difficulties.
Neuroimaging: Dr. Ahmad
The MRI showed an extra-vermis attached to the anterior lobe of the native vermis, mainly to the culmen (Figure 1A,B). The anterior superior folia of this extra-cerebellar tissue herniate superiorly through the left side of the tentorial notch (Figure 1D,E). The extra-vermis has a primary fissure and a pre-pyramidal fissure. It demonstrates a similar signal intensity to the native one on all sequences (Figure 1C,F and G). Additionally, there is an aqueductal stenosis and corpus callosum dysgenesis (Figure 1C).Reference Hanna, Marsh and Swistun1
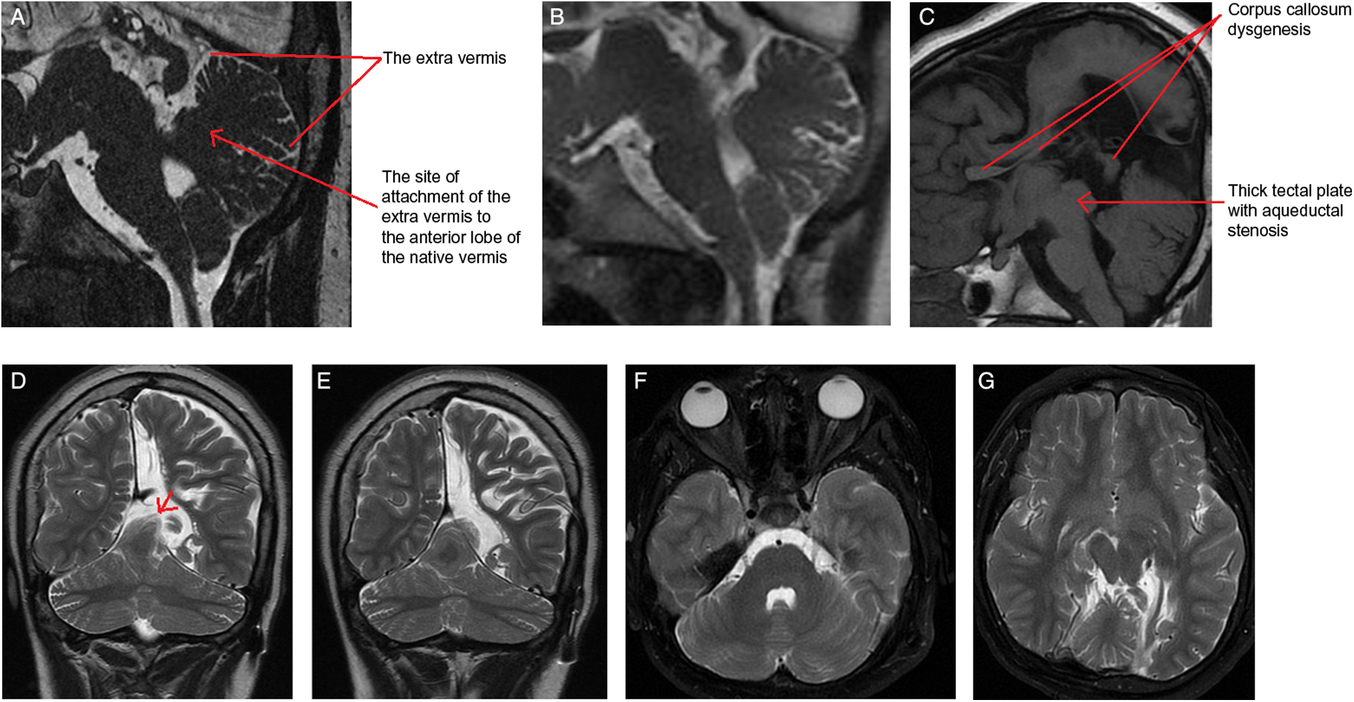
Figure 1: (A) Sagittal FIESTA image – slightly off midline – through the cerebellum demonstrates the extra vermis that is attached to the culmen of the native vermis. Notice the unique finding that the supernumerary vermis is developed from the dorsal aspect of the native vermis with a very clear site of attachment to the culmen and not from the brain stem. (B) Sagittal midline T2-weighted image shows the cerebellar vermian anomaly. (C) Sagittal T1-weighted MR image re-demonstrates the same findings, and shows the corpus callosum dysgenesis and the thick tectal plate with the resultant aqueductal stenosis. The anomalous vermis exhibits a similar signal intensity to the native one on coronal T2-weighted images (D and E). Coronal T2 weighted MRI images show the anomalous vermis herniated through the tentorium (red arrow in D). (F and G) Axial T2-weighted images show the normal morphology of the cerebellar hemispheres and re-demonstrate the extra vermis.
Discussion: Dr. Raybaud and Dr. Ahmad
The extra-vermis is believed to be the result of a developmental error that occurs at the early stages of embryogenesis.Reference Liu, Zhang and Lin2 For better understanding of this anomaly, the morphological and molecular aspects of cerebellar development are discussed here.
Morphological Aspect
The mesencephalon (midbrain (MB)) and metencephalon (upper hindbrain (HB)) contribute to form the cerebellum during the fifth week of gestation. The alar plates of the mesencephalon contribute to cerebellar vermis.Reference Hattapoglu, Hamidi, Goya, Cetincakmak, Teke and Ekici3 The rhombic lips are the primordia of the cerebellar hemispheres and the source of granule cellsReference Dun4. The rhombomeres 1–11 (r1–r11) result from the primary segmentation of the brainstem, initiated by the isthmic organizer. Rhombomeres are identified as r0 (the isthmus: the junction between MB and HB) followed by r1–r11. The cerebellum originates from r0–r1. It is generally considered that the vermis originates from the alar plate of r0 and the hemispheres from the alar plate of r1.Reference Hattapoglu, Hamidi, Goya, Cetincakmak, Teke and Ekici3
The primary fissure is the first to develop at 14 weeks. The pre-pyramidal and the secondary (post-pyramidal) fissures are seen at 16 weeks (Figure 2). The posterolateral fissure is visible at week 17.Reference Liu, Zhang and Lin2 The earliest studies of gestational age demonstrate that the ratio between the volume of the parts above and the volume of those below the primary fissure is 1:1 until the age of 16 weeks. This will be followed by rapid growth of the pyramid vermis and tuber vermis. The parts beneath the primary fissure grow faster and become larger than the parts above the fissure. Figure 3 illustrates the normal anatomy of the vermis.
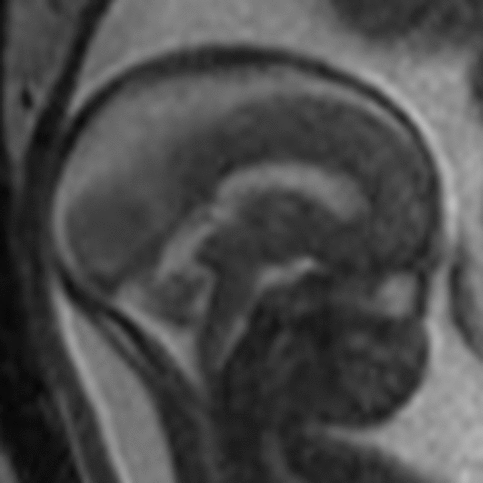
Figure 2: Midline sagittal FIESTA MRI image through the vermis in a 23-week fetus demonstrating the development of the major fissures and the vermian segments by this gestational age.
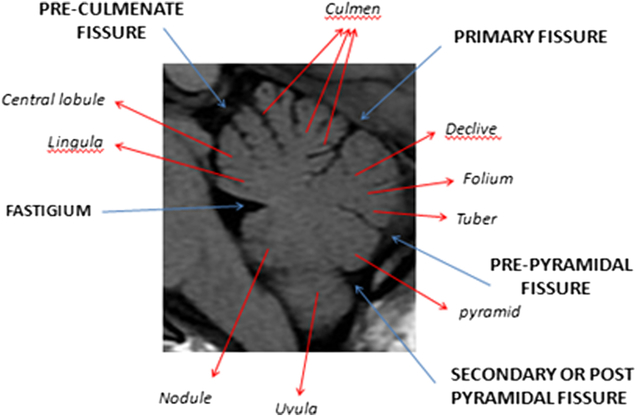
Figure 3: Annotated sagittal T1 MRI image of the cerebellum in a healthy teenage volunteer showing normal anatomy and major fissures.
Molecular Aspect: Dr. Raybaud and Dr. Nadi
Three phases can be recognized:
I. Allocation of the cerebellar anlage (CA): The CA is a product of the early embryonic segmental phase that results in rhombomeric subdivisions within the HB just after neural tube closure. The territory that generates the CA is close to the boundary (the isthmus) between the MB and the HB. The patterning in this phase is significant for the vermis evolution.Reference Butts, Green and Wingate5
II. The process of cell type allocation: Generation of neuronal diversity along the dorsoventral axis.Reference Simon, Hornbruch and Lumsden6
III. The transit amplification process. It extends up to two years postnatally in humans.Reference Butts, Green and Wingate5 We will elaborate further on phase I because the anomaly in this case is mostly due to an event that happened in the early weeks.
The CA sits between Otx and Hox domains, a region that lacks expression of Otx and Hox genes. The majority of the cerebellum arises from the rostral metencephalon and one-third of cerebellar granule cells originate from the mesencephalon.Reference Hallonet, Teillet and Le Douarin7 More precisely, it arises between the MB and HB.Reference Millet, Bloch-Gallego, Simeone and Alvarado-Mallart8 There are certain genes that determine the cerebellar territories: Otx2 at the rostral boundary, Hoxa2 at the caudal boundary, and Gbx2 which is expressed in r1, abutting Otx2. Otx2 is required to establish forebrain and MB territories, and its absence causes a rostral expansion of the cerebellar Gbx2-positive territory at the expense of MB tissue. Contrariwise, ectopic expression of Otx2 in the rostral HB transforms this region into a Gbx2-negative MB with a caudal shift in the position of the MB–HB boundary (MHB).Reference Katahira, Sato and Sugiyama9 Thus, it is an essential requirement that Otx2 is absent for cerebellar differentiation to begin. On the other hand, while Gbx2 is required for cerebellar formation,Reference Wassarman, Lewandoski and Campbell10 Hoxa2 determines the caudal limits of the cerebellum.Reference Gavalas, Davenne, Lumsden, Chambon and Rijli11
Additionally, the morphogen fibroblast growth factor 8 (Fgf8) and Gbx2Reference Tossell, Kiecker, Wizenmann, Lang and Irving12 promote cell sorting and lineage restriction at the boundary. Fgf8 signaling has a significant role in allocating isthmic territory and the origins of the vermis in the MHB. Fgf8 is expressed within the Gbx2-positive domain and essential for the survival of the isthmic region.Reference Basson, Echevarria and Ahn13 It maintains r1 as an Otx2-negative domain.Reference Foucher, Mione, Simeone, Acampora, Bally-Cuif and Houart14 The ectopic Fgf8 in the MB can induce a secondary cerebellum, whereas loss of Fgf8 expression induces vermis dysplasiaReference Basson, Echevarria and Ahn13 and expansion of the Otx2 domain.Reference Sato and Joyner15 Sprout genes are Fgf8 antagonists and regulate the levels of fibroblast growth factor signals from the r0.Reference Butts, Green and Wingate5 Mutations in sprout 2 genes (Spry2) result in expansion of the vermis.Reference Yu, Yaguchi, Echevarria, Martinez and Basson16
Interestingly, the extra vermis in our case arises from the dorsal aspect of the native vermis and not from the brain stem as it would be expected from the molecular development explained above in the cases of overexpression of Gbx2. This is likely attributed to a mutation in either Fgf8 or spry2 genes that alter the allocation of the isthmus and the origin of the extra vermis. This extra vermis showed a well-formed culmen and a central lobule as well as a primary fissure, and a pre-pyramidal fissure with no formation of the posterior lobe. This suggests that the anomaly has likely developed before the 16 weeks of gestation.
Review of Literature: Clinical Implications and Genetic Association
While no similar case of extra vermis has been described in the literature, there are few reported cases of extra cerebellar hemispheres. In 1990, Jackson et al. reported a case of duplication of a unilateral cerebellar hemisphere associated with a complex ipsilateral ear anomaly.Reference Jackson, Sadove, Weaver, Edwards and Bull17 The author traced the timing of the problem to the third week of gestation. Clinically, patient’s examination was significant to lower extremities hyper-reflexia, truncal hypotonia wide-base gait with right upper limb intentional tremors. Moreover, the patient’s IQ was low-average and he was delayed in terms of fine and gross motor skills as well as social adaptation. Another case report published in 2011 described a 16-month-old girl with a third cerebellar hemisphere and incompletely formed superior vermis.Reference Agarwal and Gathwala18 This was attributed to a mutation in genes expressed during early cerebellar development leading to an extra RL growth. The anomaly impacted the patient’s motor milestone as she was unable to stand. This child also showed mild truncal ataxia, generalized hypotonia, and diminished deep tendon reflexes. Furthermore, Hattapoğlu et al. reported a third case of supernumerary hemi-cerebellum in an adult female patient who presented with headaches and episodes of syncope.Reference Hattapoglu, Hamidi, Goya, Cetincakmak, Teke and Ekici3 Neurological exam was intact apart from a slight increase in the deep tendon reflexes. It is noteworthy that none of these three cases or our patient had language skills issues.
Several cerebellar anomalies can be associated with other congenital anomalies and there are different genetic defects responsible for them,Reference Aldinger and Doherty19 and further elaboration of various types of malformations and their genetic bases is out of the scope of this quick highlight. However, in regard to the discussed three reported cases of extracerebellar tissue, no specific genetic alterations or dedicated genetic testing was reported.Reference Hattapoglu, Hamidi, Goya, Cetincakmak, Teke and Ekici3, Reference Jackson, Sadove, Weaver, Edwards and Bull17, Reference Agarwal and Gathwala18 Jackson et al examined their patients and showed normal male chromosomes 46 XY.Reference Jackson, Sadove, Weaver, Edwards and Bull17
Conclusion
The purpose of this case review is to share an unusual cerebellar malformation with the medical community and to shed light on the potential embryological errors that could result in this anomaly. It is expected that more cases will be reported in the future with further advancement of diagnostic imaging techniques.
Authors’ declarations
All authors declare no conflict of interest in this work. There was no financial support or grant for this production. This work has never been presented before in part or whole.
Ethical Standards statement
No patient’s identification information was used. A consent form from the patient’s guardian was obtained.





