Introduction
Parkinson’s disease (PD), clinically characterized by bradykinesia, rigidity, resting tremor and impaired postural stability, is the second most common neurodegenerative disease in the elderly. Along with the rapid aging of our population, the number of PD cases has increased gradually.
Stroke and coronary artery disease (CAD, including angina and myocardial infarction) are two common vascular diseases involving cerebral and coronary artery. However, the relationship of stroke and CAD with PD remains unclear. Some,Reference Hu, Antikainen, Jousilahti, Kivipelto and Tuomilehto 1 - Reference Jain, Ton and Perera 4 but not all,Reference Simon, Chen, Schwarzschild and Ascherio 5 epidemiological studies showed that vascular risk factors (e.g., diabetes, hypertension and increase in total cholesterol) were associated with an increased risk of PD. Small cerebral vessel diseases were found to be associated with mild parkinsonian signs.Reference Hatate, Miwa and Matsumoto 6 - Reference Reitz, Trenkwalder, Kretzschmar, Roesler, Von Eckardstein and Berger 8 Rapid eye movement (REM) sleep behavior disorder, one of the common non-motor symptoms of PD, was shown to increase the risk of stroke in a community-based study.Reference Ma, Pavlova and Liu 9 These findings suggest a possible link between stroke and PD. Furthermore, cardiovascular dysautonomia was sometimes presented as another non-motor PD symptom and may occur early before motor dysfunction.Reference Goldstein 10 , Reference Chen, Zhao and Zhang 11 The possible mechanism of cardiovascular dysautonomia in PD was loss of cardiac sympathetic innervations owing to dorsal vagal nucleus degeneration.Reference Braak, Ghebremedhin, Rub, Bratzke and Del Tredici 12 These findings also suggest that CAD might be linked to PD through shared vascular risk factors and cardiac sympathetic denervation. We thus performed a community-based cross-sectional study in order to investigate whether stroke and CAD are associated with idiopathic PD in China.
Subjects and Methods
Study Population and PD Ascertainment
This two-step door-to-door study was conducted in two communities: Malu rural community from April to October 2013 and Wuliqiao urban community in 2014. The Malu rural community of Jiading district is located in the north-west rural area of Shanghai. The target population was 24,464 residents aged 50 years or older living in this area from community registry offices.Reference Ma, Hou and Tang 13 The Wuliqiao urban community of Luwan district is located downtown, and the target population was 3970 permanent residents who were recruited in our sleep and dementia cohort established in 2009 and followed up in 2011 and 2014.Reference Zhuang, Wang and Cheng 14 , Reference Ma, Qiao and Gao 15
In the first step, local doctors from medical centers of Malu and Wuliqiao community had a 1-day clinical teaching course on the evaluation of parkinsonism and essential tremor conducted by movement disorders specialists from the Department of Neurology, Rui Jin Hospital affiliated to Shanghai Jiao Tong University School of Medicine. This clinical teaching course included video presentation of symptoms of parkinsonism and essential tremor and how to retrieve the history. Under the supervision of the movement disorder specialist, the local doctor was considered qualified when they demonstrated competency to conduct the clinical examination of tremor, bradykinesia, rigidity and gait. After being trained, these local doctors visited residents aged ≥50 years from door to door and reported possible parkinsonism cases to the movement disorder specialists. In the second step, movement disorder specialists revisited these possible parkinsonism cases to make the final diagnosis. The diagnostic procedure included medical record review, neurological examinations, imaging review if possible and so on (Figures 1 and 2). The diagnosis of PD patients was based on the United Kingdom Parkinson’s Disease Society Brain Bank Clinical Diagnostic Criteria. This study was approved by the Ethical Committee of Rui Jin Hospital, with receipt of consent forms from each participant.
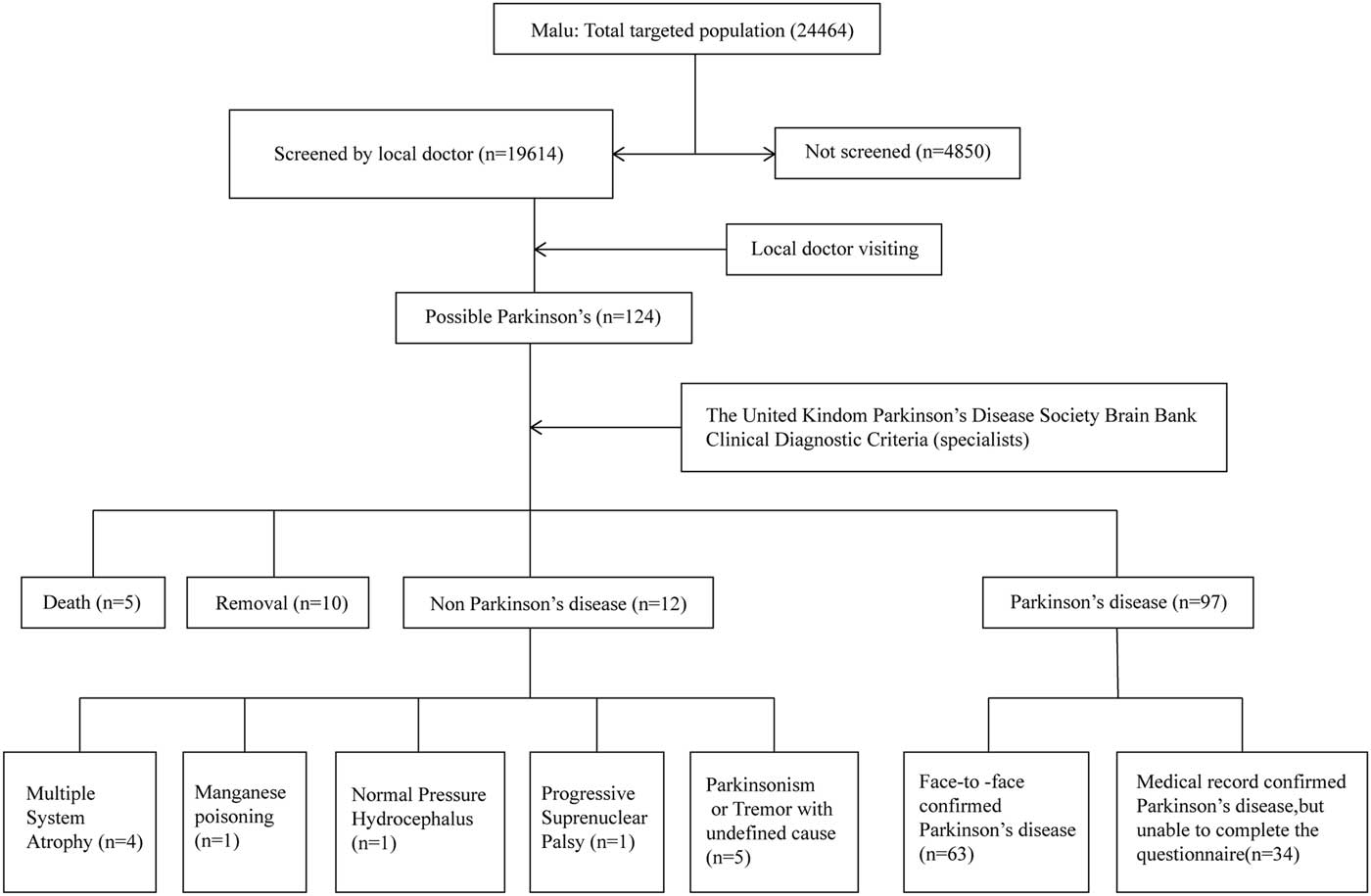
Figure 1 Flowchart of the Malu rural study.
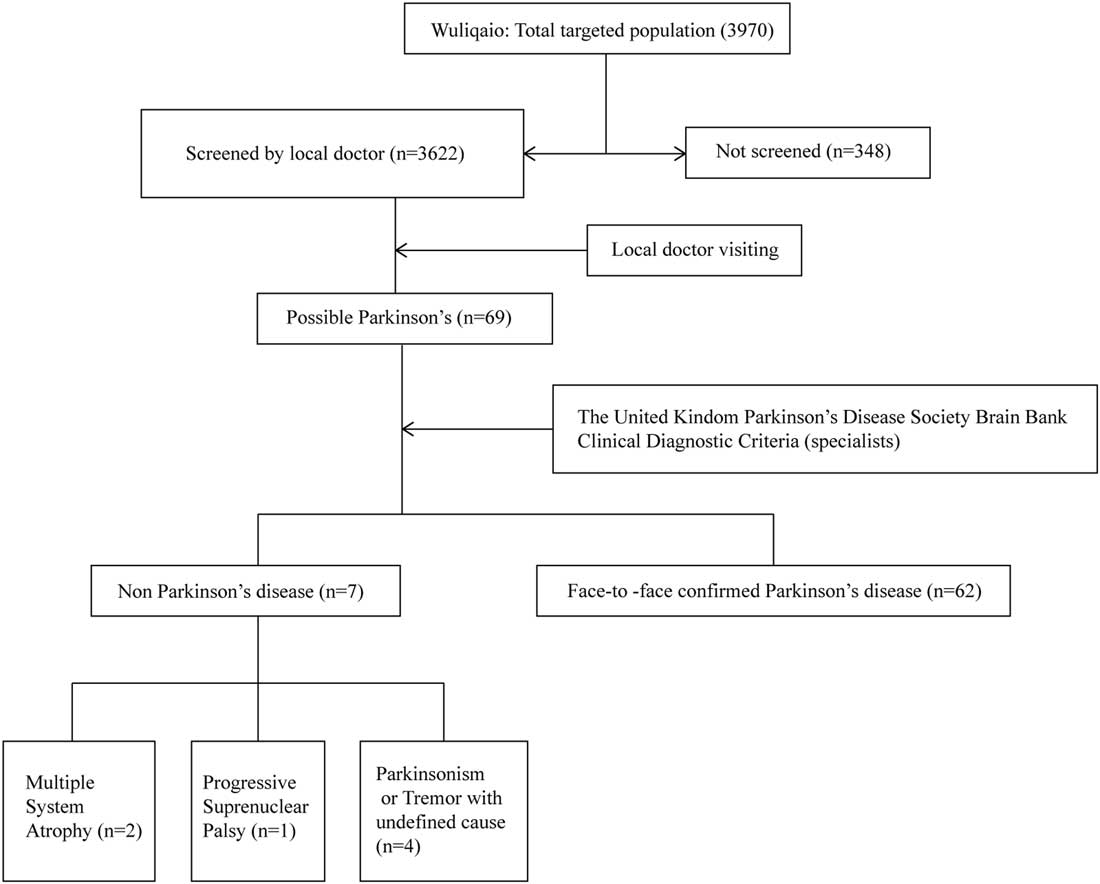
Figure 2 Flowchart of the Wuliqiao urban study.
Assessment of Coronary Artery Disease and Other Potential Cofounders
Parkinson’s disease patients and non-PD individuals willing to enter our study in the same suburb completed a questionnaire including the following information: history of CAD (yes/no), gender (female or male), age (year), weight (kilogram), height (centimeters), diabetes mellitus (yes/no), hypertension (yes/no), history of stroke (yes/no), hypercholesterolemia (yes/no), tea consumption (yes/no), smoking status (never/past smoker/current smoker: cigarettes/day, 1-19 or ≥20) and alcohol consumption (no/current: g/day, 0-9.99 or 10-19.99 or ≥20). Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared and grouped into four categories in the analysis (BMI: <18.5 or 18.5-23.9 or 24-26.9 or ≥27 kg/m2).
History of stroke and CAD was obtained by self-reporting. To increase the accuracy of diagnosis, we also recorded the hospital where the diagnosis of stroke and CAD was made and whether they saw a specialist for the diagnosis of stroke and CAD in hospitals. In the Wuliqiao community, 175 cases (36.5%) of CAD and 120 cases (35.1%) of stroke were diagnosed in third-grade class-A hospital (a high-level hospital evaluated by National Health Commission), and 302 cases (62.9%) of CAD and 220 cases (64.1%) of stroke were diagnosed in second-grade class-A hospitals (a middle-level hospital evaluated by National Health Commission). In the Malu community, more than 50% of CAD and stroke cases were diagnosed by specialists: 23 cases (24.5%) of CAD and 24 cases (24.2%) of stroke were diagnosed in third-grade class-A hospitals, and 38 cases (40.4%) of CAD and 30 cases (30.3%) of stroke were diagnosed in second-grade class-A hospitals.
Statistical Analysis
All analyses were conducted with SPSS, version 19 for Windows (SPSS Inc., Chicago, IL, USA). The characteristics of the study population (non-PD and PD) were compared by using the χ 2 test for categorical variables and Student’s t-test for continuous variables. For examining potential risk and protective factors for PD, we used multiple logistic regression models to calculate odds ratios (OR) and 95% confidence intervals (CI), adjusting for age, sex, smoking status, alcohol consumption, hypertension, diabetes mellitus, tea consumption, hypercholesterolemia and BMI.
Results
In the first step, 19,614 out of 24,464 residents (responding rate=80.2%) participated in the door-to-door interview in Malu community. A total of 124 possible PD cases were identified by local doctors. In the second step, movement disorder specialists finally confirmed the diagnosis of 97 PD patients. Among them, 63 PD patients completed the questionnaire and 34 PD patients did not complete the questionnaire for reasons such as hearing problem, unwillingness and so on (Figure 1). A total of 4483 non-PD residents from our previous dementia cohort in Malu community were included as controls and completed the questionnaire.Reference Tang, Zhou and Gao 16 For the Wuliqiao community, 3622 out of 3970 residents (responding rate=91.2%) were interviewed door-to-door and completed the questionnaire. A total of 69 possible PD cases were identified by local doctors and 62 cases were confirmed by movement disorder specialists (Figure 2). The remaining 3553 non-PD residents were included as controls in the association study. The demographic features of PD and non-PD population are listed in Table 1.
Table 1 Demographic features of Parkinson’s disease (PD) and non-PD cases

CAD=coronary artery disease.
*p<0.05.
We found that history of stroke was associated with the risk of PD both in Malu rural community (OR=6.77, 95% CI: 3.09-14.81, p<0.001) and the Wuliqiao Urban community (OR=2.58, 95% CI: 1.36-4.89, p=0.004), after adjustment for age, sex, smoking status, alcohol consumption, tea consumption, hypertension, diabetes mellitus, hypercholesterolemia and BMI (Table 2). The association of stroke and PD was consistent in the female population of Malu rural (OR=4.03, 95% CI: 1.08-15.04, p=0.04) and Wuliqiao urban communities (OR=3.15, 95% CI: 1.34-7.42, p=0.009). However, for the male population, only Malu rural community found the positive association of stroke with PD (OR=10.06, 95% CI: 3.46-29.26) (Table 2).
Table 2 Multivariate regression results for the association between coronary artery diseases (CAD), stroke and risk of having Parkinson’s disease (PD)

OR=odds ratio; CI=confidence intervals.
* Adjusted for age, sex, smoking status, alcohol consumption, tea consumption, hypertension, diabetes mellitus, hypercholesterolemia and body mass index.
** PD cases with no medication.
*p<0.05.
In addition, history of CAD was associated with PD in Malu rural community (OR=7.11, 95% CI: 3.09-16.40, p<0.001), after adjustment for age, sex, smoking status, alcohol consumption, tea consumption, hypertension, diabetes mellitus, hypercholesterolemia and BMI (Table 2). There was also a trend of association of history of CAD with PD in Wuliqiao urban community (OR=1.72, 95% CI: 0.91-3.23, p=0.09), especially in the male population (OR=2.32, 95% CI: 0.91-5.98, p=0.08). To eliminate the possible cardiovascular effects of levodopa and dopamine receptor agonist,Reference Noack, Schroeder, Heusser and Lipp 17 we restricted to PD patients without levodopa or dopaminergic agonist treatment (referred to as “drug-naïve PD cases” in the manuscript). As 34 cases of drug-naïve PD cases were found in the Malu community and only four cases in the Wuliqiao community, we only performed this analysis in the Malu rural community and the result was similar (Table 2). As the age of the individuals in the PD group was much higher than that of controls (Table 1), we performed the analysis again in an age-matched control group in both cohorts. We used PD and control group at a ratio of 1:3, matched for age, sex, hypertension, diabetes and hypercholesterolemia for individuals <90 years old, and a ratio of 1:1, matched for age and sex, for individuals ≥90 years old (not enough controls available). The results are similar to those of the whole Malu community (CAD: OR=12.72, 95% CI: 2.92-55.32, p=0.001; stroke: OR=6.26, 95% CI: 1.83-21.42, p=0.003), after adjustment for age, sex, smoking status, alcohol consumption, tea consumption, hypertension, diabetes mellitus, hypercholesterolemia and BMI. In the Wuliqiao community, CAD was found to be associated with PD (OR=2.44, 95% CI: 1.09-5.47, p=0.03), whereas stroke was not (OR=1.79, 95% CI: 0.77-4.17, p=0.18), after adjustment for age, sex, smoking status, alcohol consumption, tea consumption, hypertension, diabetes mellitus, hypercholesterolemia and BMI, which is different from the whole Wuliqiao population.
We also found that tea consumption was significantly lower in PD groups than in controls in Malu community (17.5% vs. 33.7%, p=0.007), but not in the Wuliqiao community (58.1% vs. 68.1%, p=0.09). Smoking consumption was significantly higher in PD groups than in controls in the Malu community (p=0.04), but not in the Wuliqiao community. There was no significant difference with regard to hypertension, diabetes, hypercholesterolemia and BMI, between PD and non-PD groups.
Discussion
Our study found that individuals with stroke were more likely to have PD, which was consistent with someReference de Laat, van Norden and Gons 7 , Reference Becker, Jick and Meier 18 - Reference Patel, Coutinho and Emsley 20 but not all previous studies.Reference Ghebremedhin, Rosenberger and Rub 21 , Reference Song, Kim, Cho and Chung 22 Interestingly, increased risk of stroke was also found in PD in a recent community-based study.Reference Huang, Chen and Yen 19 Cerebral small-vessel disease was shown to be associated with mild parkinsonian signs in several cohorts. Vessel lesions in certain locations were suggested, accounting for the link between stroke and PD, including frontal and parietal white matter lesion or lacunar infarcts in the thalamus.Reference Hatate, Miwa and Matsumoto 6 - Reference Reitz, Trenkwalder, Kretzschmar, Roesler, Von Eckardstein and Berger 8 Recently, small cerebral vascular changes such as microbleeds were also found to be associated with idiopathic PD.Reference Kim, Park, Kim, Ma and Kim 23 DJ-1, a gene causing familial PD, was shown to be involved in cerebral ischemia changes in in vitro and in vivo models, indicating another possible mechanism linking PD and stroke.Reference Kitamura, Watanabe and Taguchi 24 , Reference Dongworth, Mukherjee and Hall 25 However, neuropathological studies showed an inverse association of vascular lesions with PD lesions in the brains of autopsy-proven PD cases or dementia with Lewy bodies.Reference Ghebremedhin, Rosenberger and Rub 21 , Reference Schwartz, Halliday, Cordato and Kril 26 Therefore, more studies will be needed to further investigate the association between stroke and PD and to reveal the underlying mechanisms.
We also found that history of CAD might be associated with an increased risk of PD, independent of traditional risk factors such as age, male sex and smoking. Our finding was consistent with Liang’s study, which showed an increased risk of acute myocardial infarction in PD (hazard ratios=1.46).Reference Liang, Huang and Pan 27 One possible explanation is that these two diseases share a common pathogenesis mechanism. Diabetes, hypertension and hypercholesterolemia were found to be associated with PD and CAD in epidemiological studies.Reference Hu, Antikainen, Jousilahti, Kivipelto and Tuomilehto 1 - Reference Xu, Park and Huang 3 , Reference Franklin, Gokhale and Chow 28 It was possible that these vascular risk factors might contribute to PD and CAD through the same mechanisms. Another explanation for the relationship between CAD and PD is cardiac sympathetic denervation in PD. Cardiac sympathetic nerve is quite sensitive to ischemia.Reference Guertner, Klepzig and Maul 29 Chronic cardiac sympathetic denervation might aggravate the cardiac ischemia by sustained elevation of end-diastolic pressure after ischemia.Reference Lavallee, Amano, Vatner, Manders, Randall and Thomas 30 In PD with orthostatic hypotension, chronic hypotension might result in exaggerated response to exogenously given adrenergic receptor agonists, a phenomenon called “denervation supersensitivity”.Reference Senard, Valet and Durrieu 31 , Reference Imrich, Eldadah and Bentho 32 Thus, it is possible that cardiac sympathetic denervation contributes to the risk of CAD by aggravating the damage to myocardial ischemia through abnormal pressure change after ischemia. Certainly, more studies need to be performed to confirm this assumption in the future.
Although anti-parkinsonian medications such as levodopa were found to exert cardiovascular side effects, our further analysis including only PD without levodopa or dopamine receptor agonist revealed similar results. These findings suggested that the association of PD and CAD was not mediated by medication such as levodopa or dopamine agonist. However, as the sample size of drug-naïve PD cases was extremely small, the results should be read cautiously.
Caffeine consumption and smoking were shown to be protective for PD in various epidemiological studies.Reference Ma, Liu, Neumann and Gao 33 , Reference Ascherio and Schwarzschild 34 In our study, tea consumption was lower in PD groups than in controls in both cohorts, but only reached statistical significance in the Malu community. However, smoking consumption was significantly higher in PD groups than in controls in the Malu community, but not in the Wuliqiao community. The negative or even inverse findings might be attributed to the small sample size, methodological difference and so on.
The strengths of our study are a door-to-door survey avoiding referral bias and PD diagnosis by movement disorders specialists. However, there are still some limitations. First, there were only 159 confirmed PD patients (97 from Malu and 62 from Wuliqiao) found in our door-to-door study. Estimated prevalence of PD in Shanghai elderly ≥50 years older will be 0.5% in the rural community and 1.7% in the urban community, which was quite low compared with other epidemiological studies for the rural community.Reference Zhang, Roman and Hong 35 Although we trained the local doctors to screen PD patients, there is a high possibility that they lost some PD patients, especially for rural local doctors. Second, history of stroke and CAD was obtained by self-reporting. There might be some inaccuracy in the information provided by self-reporting history. More than 90% of CAD and stroke cases in the Wuliqiao community were diagnosed by specialists and more than 50% of CAD and stroke cases in the Malu community were diagnosed by specialists, indicating a comparable reliability of CAD and stroke diagnosis in our cohorts. However, self-recollected information of CAD might render a recall bias, as well as an understanding of the different diagnosis and educational levels, which may play an important role in our results. Third, our study was retrospective and performed in Chinese Shanghai area, and thus the results represent the population in Shanghai.
In conclusion, we found that history of stroke and CAD was associated with PD in two Chinese population-based cohorts. These findings suggest that vascular disease might play some role in the pathogenesis of PD. Therefore, it is still important to investigate the mechanism linking stroke and CAD with PD, and probably prevent or treat PD by modifying vascular factors in the future.
Acknowledgments
This study was supported by National Key R&D Program of China (2016YFC1306000). The authors thank Dr. Lifang Zhu, Dr. Yanqing Lu and all other doctors from Malu Medical Center for their support of our epidemiology study.
Disclosures
QL, HT, SC and JM received the grant from National Key R&D Program of China (grant no. 2016YFC1306000). CW has nothing to disclose.
Statement of Authorship
QL collected data, performed the statistical analysis and drafted the manuscript. CW helped to collect data. HT made the diagnosis of PD. SC double-checked the diagnosis of PD. JM designed the study, double-checked the statistical analysis and revised the manuscript.






