The classical tremor of Parkinson’s disease (PD) occurs during rest and is usually suppressed during movement. Holmes’ tremor (HT) is a rare symptomatic movement disorder characterized by tremor at rest, enhanced by posture and further exacerbated by kinetic action, which occurs after a variety of lesions involving the brainstem and thalamus. The clinical phenomenology of HT is complex and may be accompanied by symptoms of ataxia, dystonia, ophthalmoplegia and bradykinesia.Reference Deuschl, Bain and Brin 1 - Reference Raina, Cersosimo and Folgar 3 Studies suggest that combined damage to the cerebello-thalamic and nigrostriatal systems is required for the genesis of HT.Reference Deuschl, Bain and Brin 1 - Reference Deuschl and Bergman 4 Here, we report a patient with prior cerebellar dysfunction who subsequently developed bilateral PD with both HT and classical parkinsonian rest tremor.
In 2014 an 82-year-old patient presented with a two-year history of tremor in both hands. His medical history included vertebrobasilar stroke in 1989, at the age of 57, when cranial magnetic resonance imaging (MRI) revealed a left temporo-occipital hemorrhagic infarction, ischemic lesion in the right pontomedullary region, and a small ischemic lesion in the right cerebellar hemisphere. This stroke left him with a right homonymous hemianopia and a slight cerebellar ataxia on the right side, which had lasted for 25 years and forced him to become left-handed.
In 2014 clinical examination prior to beginning dopaminergic treatment demonstrated a bilateral tremor with inconsistent characteristics in his upper limbs: a coarse, irregular, low frequency resting tremor in the right arm which was accentuated by posture and further increased by goal-directed movements; on the left side, he had classic parkinsonian rest hand tremor without postural or intention tremor (video 1, segments 1-4). In addition, the patient had right homonymous hemianopia examined by confrontation, dystonic posturing in the right hand, mild hypomimia, chin tremor, and bilateral mild rigidity and bradykinesia (video 1, segment 5). Electromyographic recordings revealed alternating contraction of agonist and antagonist muscles of the left upper limb during the resting limb position at a frequency of less than 6 Hz and an irregular tremor at rest, at lower frequencies in the right arm (Figure 1). There was no co-contraction of the agonist-antagonist muscles in the right upper limb. Cranial MRI revealed left parieto-temporal atrophy with left ventricular ectasia, encephalomalacia in the left posterior cerebral artery territory, small infarction in the right cerebellum and lacunar infarction in the left thalamus (Figure 2 A-D). Single-photon emission computed tomography with I123 ioflupane (DaTSCAN) demonstrated a marked dopaminergic deficiency in the bilateral striatum (figure 3). PD was diagnosed and the patient received levodopa, reaching a final dose of 750 mg/day with positive response of the three components of HT. Rest tremor in the left arm, chin tremor and other parkinsonian symptoms (rigidity and bradykinesia) were slightly improved.
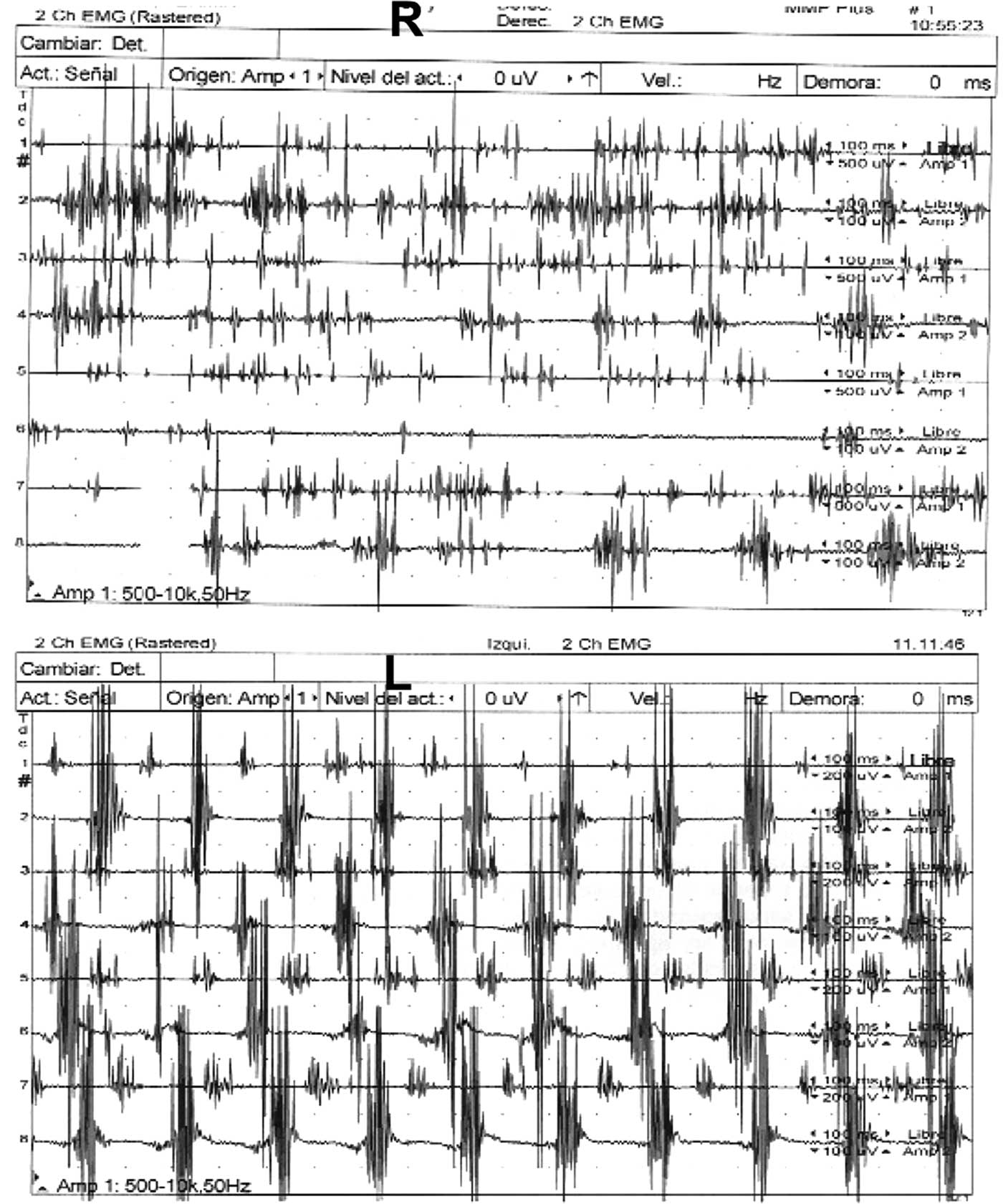
Figure 1 Electromyographic recordings of the tremor of both hands.
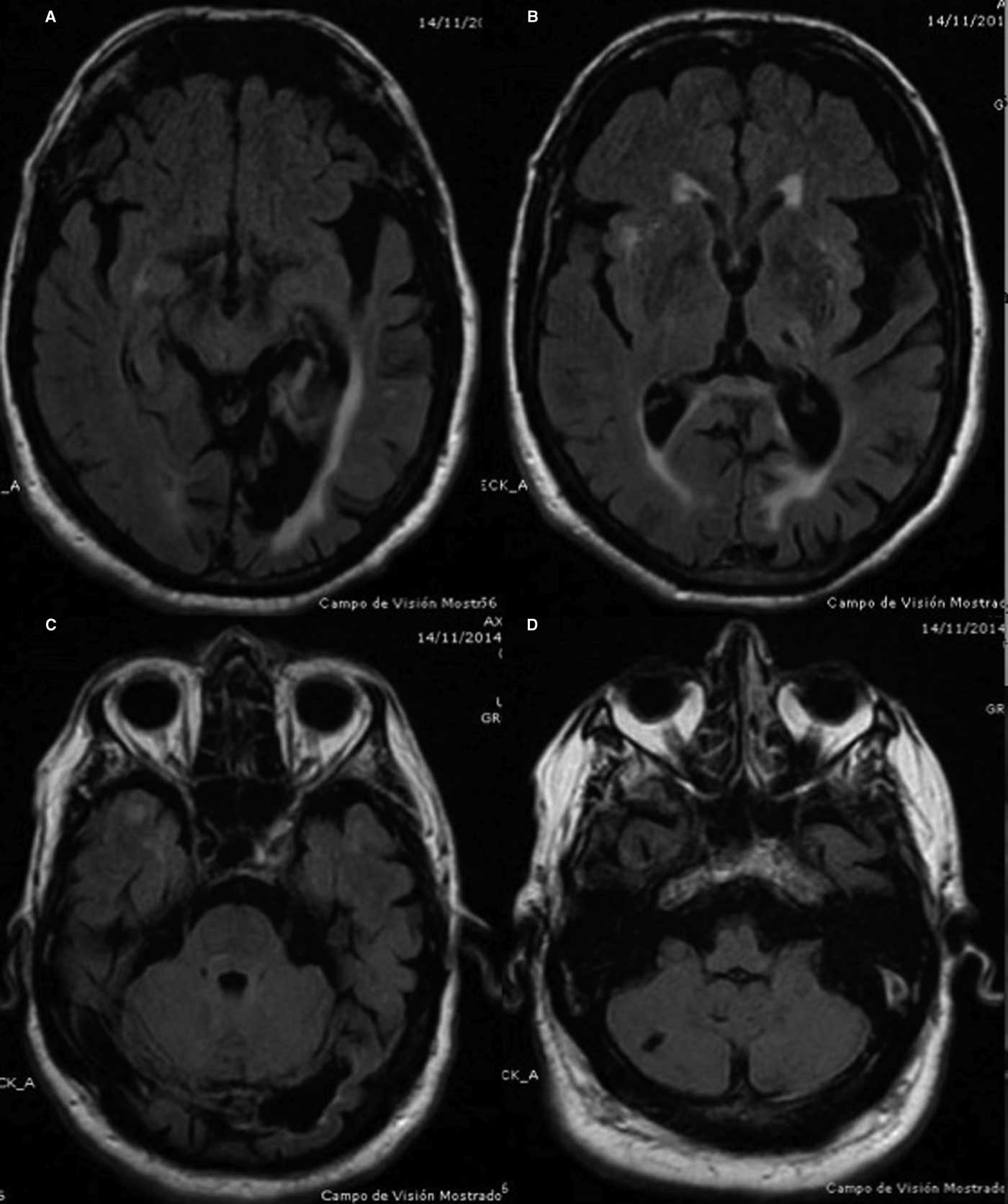
Figure 2 Axial FLAIR cranial MRI images.

Figure 3 Single-photon emission computed tomography with I123 ioflupane (DaTSCAN) images.
The right tremor in our case met the criteria for HT defined by the Ad Hoc Scientific Committee of the Movement Disorders Society:Reference Deuschl, Bain and Brin 1 rest and intention tremor, sometimes postural tremor of low frequency, with irregular amplitude, and usually arrhythmic. HT is a symptomatic tremor that occurs after a variety of lesions centered on the brainstem/cerebellum and thalamus, although in some cases no lesion can be demonstrated.
The presence of a pre-existing brain lesion may change the clinical presentation of PD. Three cases presenting with atypical clinical features of PD associated with the mass effect of a significantly enlarged pre-existing Virchow-Robin space have been reported.Reference Mestre, Armstrong, Walsh, Dakheel, Moro, Stoessl and Lang 5 Our case had a pre-existing cerebellar dysfunction, and the HT developed after the appearance of PD. A few patients have been reported with HT caused by pre-existing cerebellar dysfunction and subsequent nigrostriatal deficit.Reference Deuschl and Bergman 4 , Reference Deuschl, Wilms, Krack, Wurker and Heiss 6 Another case report described a patient with PD who developed HT after acute cerebellar stroke.Reference Kim, Koo, Kim and Oh 7
These findings provide strong clinical evidence that separate lesions in cerebellar and nigrostriatal pathways, with different etiologies and occurring at different times, also induce HT. In our patient, lacunar lesion of the cerebellum or the thalamus cannot explain the significant damage through the cerebello-thalamic pathways; however, as mentioned above, in some cases of HT no lesion can be demonstrated. A possible explanation for the occurrence of HT in our patient is that there was a pre-existing cerebello-thalamic dysfunction due to the vertebrobasilar stroke and a subsequent nigrostriatal dysfunction due to PD. These systems also have lesions along the further projections of their fiber tracts, and so the HT might also be caused by lesions in other locations (for example, at multiple cortical sites)Reference Deuschl, Bain and Brin 1 .
Recent reports have speculated about the pathophysiological heterogeneity of HT. This is supported by DaTSCAN studies that show only minimal differences between the two sides, with slightly poorer uptake contralateral to the HT.Reference Gajos, Bogucki and Schinwelski 2 These studies raise the question of whether nigrostriatal pathway damage is crucial for the phenomenology of this condition. Levodopa responsiveness is also regarded as a marker of nigrostriatal involvement in HT: given that some patients fail to respond to levodopa, it has been speculated that variations in pathophysiology may explain the different responses to drugs.Reference Gajos, Bogucki and Schinwelski 2 , Reference Raina, Cersosimo and Folgar 3 , Reference Deuschl, Wilms, Krack, Wurker and Heiss 6 , Reference Kim, Koo, Kim and Oh 7 In our case, both the DaTSCAN study and the improvement after levodopa therapy suggest that nigrostriatal pathway damage is involved.
Finally, some studies of the cerebral interactions between motor planning and PD tremor at rest suggest that this tremor and voluntary movement execution arise from similar oscillations in the motor cortex, which may explain why they do not occur simultaneously.Reference Helmich, Hallet, Deuschl, Toni and Bloem 8 Our patient had a cerebellar dysfunction before the development of PD and presented resting tremor on both sides but with different frequencies and with an additional postural and intention tremor on the same side as the cerebellar dysfunction. Then, the observation that tremor at rest is no longer inhibited at movement onset when the cerebellum is malfunctioning suggests that the cerebellum may modulate the frequency and amplitude of PD tremor by treating it as if it were a voluntary motor behavior.Reference Deuschl and Bergman 4 ,6, Reference Helmich, Hallet, Deuschl, Toni and Bloem 8
In conclusion, our case supports the hypothesis that the combined damage of cerebello-thalamic and nigrostriatal systems is required for the genesis of HT. Our findings also suggest that the cerebellum plays a role in the characteristics of PD tremor and is involved in the suppression of resting tremor during voluntary movements.
Disclosures
Asunción Ávila, Nuria Caballol, and Martí Martí do not have anything to disclose.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2016.451





