Introduction
Subarachnoid hemorrhage (SAH), a subtype of hemorrhagic stroke, accounts for approximately 5% of all strokes and has an overall annual incidence of 2–22 per 100,000 persons.Reference Connolly, Rabinstein and Carhuapoma 1 , Reference de Rooij, Linn, van der Plas, Algra and Rinkel 2 It is most commonly caused by a ruptured brain aneurysm and carries a high risk of morbidity and mortality. Known risk factors for an SAH include: female sex, hypertension, smoking, cocaine use, personal or family history of SAH, polycystic kidney disease, and alcohol abuse.Reference Connolly, Rabinstein and Carhuapoma 1 SAHs tend to occur in a younger population (mean age 55 years) compared to the more common ischemic stroke, and thus complications may have a devastating impact on patients’ productive life years. Although mortality rates have declined from approximately 60% in the 1970s to 20–30% today as a result of early aneurysm repair and improvements in aggressive management of complications, roughly half of SAH survivors still incur some form of disability.Reference Connolly, Rabinstein and Carhuapoma 1 Complications may account for up to 25% of deaths following an SAH, and include, but are not limited to, aneurysm rebleeding, delayed cerebral ischemia (DCI) with consequential neurological and functional deficits, hydrocephalus, hyponatremia, fever, and seizures.Reference Connolly, Rabinstein and Carhuapoma 1 High-quality evidence guiding the management of many of these complications is lacking.Reference Diringer, Bleck and Claude Hemphill 3 , Reference Marigold, Günther, Tiwari and Kwan 4
Seizures and epilepsy are important complications following an SAH. The rates of seizures and post-SAH epilepsy have been reported to range from 0 to 31% and 3.1 to 13%, respectively (Table 1). Seizures are a matter of concern for their potential association with such adverse outcomes as aneurysm rebleeding and additional cerebral injury, contributing to worsened long-term functional outcomes and increased mortality. Controversy exists in guiding decisions on the need for seizure prophylaxis in all patients versus only treating those who have actually experienced seizures.Reference Lanzino, D’Urso and Suarez 5 In addition, the choice of antiepileptic drug (AED) therapy in SAH is a subject of debate due to a lack of quality prospective data to guide such a decision.Reference Marigold, Günther, Tiwari and Kwan 4 The purpose of this review is to provide an up-to-date summary of the evidence on the incidence and outcomes of seizures following an SAH as well as the use of different AEDs following an SAH in order to evaluate the need for seizure prophylaxis, the choice of AEDs, and their dosing considerations in SAH patients.
Table 1 Summary of included studies

Prophylaxis (P) refers to patients who were given AED therapy prior to any known seizure activity (including onset seizures). Treatment (T) refers to AED therapy initiated after any seizures (including onset) occurred.
ADR=adverse drug reaction; AED=antiepileptic drug; BID=twice daily; BP=blood pressure; CBZ=carbamazepine; DCI=delayed cerebral ischemia; DRS=disability rating scale; FosPHT=fosphenytoin; GOS=Glasgow Outcome Scale; GOSE=Glasgow Outcome Scale extended; ICH=intracranial hemorrhage; ICU=intensive care unit; LD=loading dose; LEV=levetiracetam; LFT=liver function test; LOS=length of stay; MD=maintenance dose; mRS=modified Rankin Scale; NCSE=nonconvulsive status epilepticus; NSICU=neuroscience intensive care unit; PHB=phenobarbital; PHT=phenytoin; PO=by mouth; QoL=quality of life; SE=status epilepticus; TID=three times daily; VPA=valproic acid or valproate; WFNS=World Federation of Neurological Surgeons.
Methods
Search Strategy
A literature search of PubMed, Medline, EMBASE, and the Cochrane Library until 1 February 2017 was performed. The following keywords were used to complete the search: “subarachnoid h(a)emorrhage,” “SAH,” and all anticonvulsants, including: “acetazolamide,” “brivaracetam,” “carbamazepine,” “clobazam,” “clonazepam,” “clorazepate,” “diazepam,” “divalproex,” “eslicarbazepine,” “ethosuximide,” “felbamate,” “fosphenytoin,” “gabapentin,” “ketamine,” “lacosamide,” “lamotrigine,” “levetiracetam,” “lorazepam,” “midazolam,” “oxcarbazepine,” “paraldehyde,” “pentobarbital,” “perampanel,” “phenobarbital,” “phenytoin,” “pregabalin,” “primidone,” “progabide,” “propofol,” “retigabine,” “ezogabine,” “rufinamide,” “stiripentol,” “sultiame,” “sulthiame,” “tiagabine,” “topiramate,” “valproic acid,” “valproate,” “vigabatrin,” “zonisamide,” “anticonvulsant,” “antiepileptic,” or “AED.” Keywords were selected by both authors to cover any use of all currently available antiepileptic drugs in patients with an SAH.
Study Selection
Human studies focusing on seizures and the use of AEDs following an SAH were included. Titles and abstracts were screened to exclude nonhuman studies, non-English studies that could not be easily translated into English using an online translator tool, and nonrelevant studies. Studies that included exclusively subjects with traumatic brain injury, brain malignancy, or ischemic stroke were also excluded. Only original primary research was included. Commentaries, editorials, conference abstracts, and reviews were also excluded. Then, the full texts of the selected articles were assessed for inclusion in our review. Lastly, a manual search for additional relevant studies was performed by analyzing the reference lists of the selected studies. In case of any discrepancies between the reviewers, further discussion was undertaken to reach a consensus.
Data Collection
Data collected included study type, year of publication, number of subjects with an SAH, seizure incidence, percentage of patients given AEDs, individual AEDs used, AED dosing, duration, pharmacokinetic characteristics, adverse reactions, outcomes assessed, and main study findings. The outcome data collected were mortality, rebleeding, disability at three months, and, when available, post-SAH epilepsy.
Results
The initial search of the databases identified 2,238 records. After removal of duplicate records, exclusion of nonhuman and non-English-language studies, and title and abstract screening, 101 records were identified. Following full-text screening, a total of 37 studies was included (Table 1). The majority of these studies were observational, 24 of which were retrospective. A total of 16 studies focused primarily on seizure incidence and outcomes, while 21 evaluated at least one aspect of AED use, including choice of AED, efficacy, comparison with other AEDs, adverse effects, dosing, and pharmacokinetics. Based on this, the available evidence was considered low or very low using the GRADE working group criteria.Reference Guyatt, Oxman and Akl 6 As a result of the variability of the included studies’ objectives, the outcome data assessed varied across studies (Table 1).
Discussion
Incidence and Impact of Seizures Following SAH
Seizures following an SAH may occur at onset, during a hospital stay (early or late), and/or after discharge (epilepsy). However, the definition of seizures and their temporal relation to the initial hemorrhage vary among studies, thus impeding accurate identification of seizure incidence. Most authors have defined onset seizures as occurring within 12 to 24 hours of the initial hemorrhage.Reference Choi, Chun, Yi, Ko, Kim and Kim 7 - Reference Lin, Dumont and Lieu 14 “Early” seizures have been defined as seizures occurring within 3 days, 7 days, and between 1 and 14 days from onset in three different studies.Reference Lin, Chang and Chang 11 , Reference Murphy-Human, Welch, Zipfel, Diringer and Dhar 15 , Reference Huttunen, Kurki and von Und 16 Definitions of “late” seizures were equally variable. For example, late seizures have been defined as occurring between 24 hours and 6 weeks of the initial hemorrhage,Reference Butzkueven, Evans and Pitman 13 more than 1 week following surgery for an SAH,Reference Choi, Chun, Yi, Ko, Kim and Kim 7 12 or more hours after hemorrhage but before surgical repair,Reference Hart, Byer, Slaughter, Hewett and Easton 10 more than 14 days after the initial hemorrhage,Reference Lin, Chang and Chang 11 , Reference Sundaram and Chow 17 or as any seizures occurring after discharge. Alternatively, some have classified seizures in temporal relation to surgical repair, to the patient’s hospital admission, or to initiation of AED therapy,Reference Choi, Chun, Yi, Ko, Kim and Kim 7 , Reference Lin, Dumont and Lieu 14 , Reference Murphy-Human, Welch, Zipfel, Diringer and Dhar 15 , Reference Baker, Prestigiacomo and Solomon 18 - Reference Raper, Kokabi and McGee-Collett 21 whereas others have simply reported an overall incidence of any seizures that occurred during the patient’s hospital stay.Reference Fung, Balmer and Murek 9 , Reference Naidech, Kreiter and Janjua 22 - Reference Taylor, Heinrichs, Janzen and Ehtisham 28 Furthermore, the definition of what constitutes a seizure was inconsistent among studies. Many identified seizures as focal or generalized rhythmic jerking or tonic–clonic contractions, as reported by bystanders, family members, or medical personnel.Reference Choi, Chun, Yi, Ko, Kim and Kim 7 , Reference Fung, Balmer and Murek 9 , Reference Butzkueven, Evans and Pitman 13 , Reference Lin, Dumont and Lieu 14 , Reference Sundaram and Chow 17 , Reference Rhoney, Tipps, Murry, Basham, Michael and Coplin 19 , Reference Karamchandani, Fletcher, Pandey and Rajajee 20 This allows for potential subjective interpretation of the event. It has been suggested that nonepileptic tonic posturing occurring at the SAH ictus as a result of elevated intracranial pressure may often be incorrectly mistaken for a seizure, which would produce an overestimation of the incidence of onset seizures.Reference Dennis, Claassen, Hirsch, Emerson, Connolly and Mayer 29 , Reference Haines 30 Establishment of standardized definitions in SAH is underway, and this will benefit future research by facilitating comparison of results across the literature.Reference Jaja, Attalla and Macdonald 31
Onset Seizures
The prevalence of onset seizures has been reported to range from 2.8 to 19%,Reference Choi, Chun, Yi, Ko, Kim and Kim 7 - Reference Lin, Dumont and Lieu 14 , Reference Raper, Kokabi and McGee-Collett 21 , Reference Naidech, Kreiter and Janjua 22 , Reference Shah and Husain 32 with the majority occurring within 1–3 hours post-hemorrhage.Reference Lin, Chang and Chang 11 , Reference Butzkueven, Evans and Pitman 13 , Reference Lin, Dumont and Lieu 14 Several studies have yielded conflicting findings regarding the association between onset seizures and negative outcomes. One study reported an association between onset seizures and increased risk of late seizures,Reference Butzkueven, Evans and Pitman 13 while several others have observed no such association.Reference Choi, Chun, Yi, Ko, Kim and Kim 7 , Reference Byrne, Boardman, Ioannidis, Adcock and Traill 8 , Reference Lin, Dumont and Lieu 14 , Reference Murphy-Human, Welch, Zipfel, Diringer and Dhar 15 , Reference Rhoney, Tipps, Murry, Basham, Michael and Coplin 19 Onset seizures have also been reported to be associated with disability at 6 weeks post-SAH, persistent neurological deficits more than 2 years post-SAH, rebleeding, and mortality.Reference Pinto, Canhao and Ferro 12 - Reference Lin, Dumont and Lieu 14 In contrast, others have not found any link between onset seizures and rebleeding, residual deficits (though the authors’ definition of “deficit” vs. “no deficit” was unclear), or mortality.Reference Hart, Byer, Slaughter, Hewett and Easton 10 In addition, another study found that almost 70% of patients with onset seizures actually had favorable outcomes at follow-up, despite having identified an association between onset seizures and poor World Federation of Neurological Surgeons (WFNS) grade on admission.Reference Fung, Balmer and Murek 9 The authors hypothesized that onset seizures distort patients’ clinical picture on admission, resulting in an erroneously poor WFNS grading. Some researchers have proposed that onset seizures are merely a marker of SAH severity and that the associated deficits occur as a result of degree of acute insult, not due to seizure activity.Reference Butzkueven, Evans and Pitman 13 , Reference Lin, Dumont and Lieu 14 Study and population heterogeneity, variability in defining and identifying seizures, and small sample sizes with correspondingly low event rates may be potential explanations for the conflicting findings.
Post-Admission Seizures
The overall reported rate of postadmission seizures in SAH have ranged from 0 to 31%.Reference Choi, Chun, Yi, Ko, Kim and Kim 7 , Reference Fung, Balmer and Murek 9 - Reference Lin, Chang and Chang 11 , Reference Lin, Dumont and Lieu 14 , Reference Murphy-Human, Welch, Zipfel, Diringer and Dhar 15 , Reference Sundaram and Chow 17 , Reference Karamchandani, Fletcher, Pandey and Rajajee 20 , Reference De Marchis, Pugin and Meyers 24 - Reference Ibrahim, Fallah and Macdonald 26 , Reference Taylor, Heinrichs, Janzen and Ehtisham 28 , Reference Dennis, Claassen, Hirsch, Emerson, Connolly and Mayer 29 , 33 - Reference Yoon, Joo, Kim, Hong and Chung 37 The highest reported rate (31%) was observed by Dennis et al.Reference Dennis, Claassen, Hirsch, Emerson, Connolly and Mayer 29 in patients with nonconvulsive seizures detected by electroencephalogram (EEG). On the other hand, the study that did not report any seizures excluded patients with onset seizures and included only patients with SAH Hunt–Hess grades of I to III.Reference Yoon, Joo, Kim, Hong and Chung 37 This finding raises the question of whether AED prophylaxis is beneficial in low-grade SAH patients presenting without onset seizures. Several studies have reported worse outcomes with postadmission seizures. One study of 402 patients, more than half of whom had a Hunt–Hess grade of IV or V, identified a relationship between increased seizure burden (hours of epileptic activity detected on EEG) and unfavorable cognitive and functional outcomes. Every 1 hour of seizure activity was associated with 1.1 times higher odds of disability or mortality at 3 months.Reference De Marchis, Pugin and Meyers 24 These findings encourage the use of cEEG in poor-grade SAH patients with risk factors for nonconvulsive status epilepticus (NCSE), especially when NCSE has been reported to be associated with very poor prognosis and high mortality rates (82–100%).Reference Dennis, Claassen, Hirsch, Emerson, Connolly and Mayer 29 , Reference Little, Kerrigan and McDougall 35 , Reference Claassen, Peery and Kreiter 38 Associations between postadmission seizures and aneurysm rebleeding,Reference Hart, Byer, Slaughter, Hewett and Easton 10 longer hospital length of stay (LOS),Reference Murphy-Human, Welch, Zipfel, Diringer and Dhar 15 , Reference Rhoney, Tipps, Murry, Basham, Michael and Coplin 19 and mortality at 12 months have also been observed.Reference Claassen, Peery and Kreiter 38 On the other hand, some studies have found no association between seizures and poor Glasgow Outcome Scale (GOS) score at 1 year post-SAH,Reference Lin, Chang and Chang 11 or poor functional outcomes.Reference Huttunen, Kurki and von Und 16 , Reference Karamchandani, Fletcher, Pandey and Rajajee 20
Epilepsy
Post-SAH epilepsy is an important outcome in the SAH population given its link to functional disability, poor quality of life, and increased anxiety.Reference Claassen, Peery and Kreiter 38 The reported incidence has ranged from 3.1 to 13%.Reference Choi, Chun, Yi, Ko, Kim and Kim 7 , Reference Lin, Dumont and Lieu 14 , Reference Huttunen, Kurki and von Und 16 , Reference Claassen, Peery and Kreiter 38 Although Huttunen et al.Reference Huttunen, Kurki and von Und 16 found that acute seizures (within 7 days of initial hemorrhage) were a risk factor for development of epilepsy, the remainder of the studies did not find a similar association,Reference Choi, Chun, Yi, Ko, Kim and Kim 7 , Reference Lin, Chang and Chang 11 , Reference Lin, Dumont and Lieu 14 , Reference Hasan, Schonck, Avezaat, Tanghe, van Gijn and van der Lugt 36 , Reference Claassen, Peery and Kreiter 38 suggesting that long-term AED therapy following SAH may not be necessary.
Risk Factors of Seizures Following SAH
Several factors have been found to be associated with seizures following an SAH. Table 2 summarizes the risk factors for seizures and epilepsy in SAH patients.
Table 2 Number of studies identifying risk factors for different types of seizures post-SAH
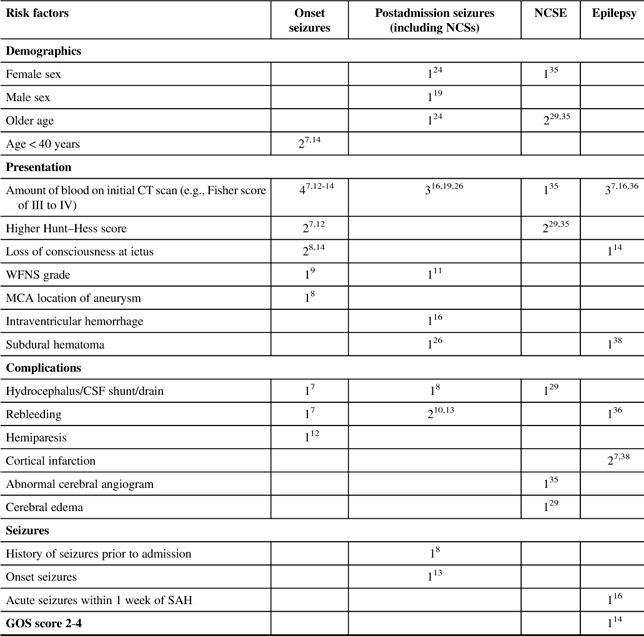
CSF=cerebral spinal fluid; CT=computerized tomography; GOS=Glasgow Outcome Scale; ICH=intracranial hemorrhage; MCA=middle cerebral artery; NCSE=nonconvulsive status epilepticus; NCSs=nonconvulsive seizures; WFNS=World Federation of Neurological Surgeons scale.
Is Seizure Prophylaxis Needed?
Historically, most patients presenting with an SAH would receive AED therapy in an effort to avoid the apparent harmful effects of seizures.Reference Choi, Chun, Yi, Ko, Kim and Kim 7 , Reference Butzkueven, Evans and Pitman 13 , Reference Lin, Dumont and Lieu 14 , Reference Rhoney, Tipps, Murry, Basham, Michael and Coplin 19 , Reference Rosengart, Huo and Tolentino 39 However, growing evidence of poor outcomes with AED therapy has made clinicians more hesitant to initiate AED prophylaxis, and this was reflected in the most recent SAH guidelines.Reference Connolly, Rabinstein and Carhuapoma 1 , Reference Rosengart, Huo and Tolentino 39 For example, one study that found poor long-term outcomes in patients who developed epilepsy by 12 months post-SAH (94% of whom were on AED therapy) suggested that the negative outcomes could be a result of the epilepsy itself or of the adverse effects from the AEDs used to treat the epilepsy.Reference Claassen, Peery and Kreiter 38 Additionally, two retrospective studies did not find a difference in seizure incidence with AED prophylaxis.Reference Raper, Kokabi and McGee-Collett 21 , Reference Panczykowski, Pease and Zhao 40 Therefore, the role of AEDs in preventing seizures in patients with an SAH remains unclear, especially considering the shortfalls of retrospectively gathered observational data, which comprises the majority of the findings. Therefore, weighing the benefits of preventing detrimental outcomes associated with seizures against the risks of adverse effects of AEDs and their potential long-term sequelae might need to be considered. Generally, seizure prophylaxis is not warranted given the currently available evidence. However, an awareness of the risk factors for seizures (Table 2) may aid in determining which patients are most likely to benefit from seizure prophylaxis following an SAH. In addition, identification of patients with risk factors for epilepsy may aid in deciding about in which patients long-term AED therapy might be indicated.
Choice of Antiepileptic Drugs for Seizures Following an SAH
The choice of initial AED therapy in SAH is a subject of great debate due to a lack of quality prospective data to guide such a decision.Reference Marigold, Günther, Tiwari and Kwan 4 Though most studies have specified which AEDs were used, some have reported outcomes in general terms of AED-treated versus non-AED-treated patients. In studies that specified AED use, selection of AED has been variable. Phenytoin, fosphenytoin, phenobarbital, levetiracetam, valproic acid, carbamazepine, zonisamide, and topiramate have been reported in the SAH population (Table 1). The advantages must be weighed against the disadvantages unique to each AED in the context of an SAH when selecting appropriate AED therapy. Phenobarbital use has generally fallen out of favor as a first-line agent, as newer, safer, and less-sedating AEDs have been developed. Carbamazepine has been used in a few studies,Reference Choi, Chun, Yi, Ko, Kim and Kim 7 , Reference Lin, Dumont and Lieu 14 , Reference Rhoney, Tipps, Murry, Basham, Michael and Coplin 19 but the evidence for its use is scarce, and it is not easily or quickly loaded in emergency situations.Reference Patsalos, Berry and Bourgeois 41 Phenytoin, levetiracetam, and valproic acid appear to be the most-studied agents in the SAH literature, and these will be discussed in detail below.
Phenytoin
Phenytoin, a hydantoin group compound, was one of the first AEDs discovered and approved for use in 1953. Phenytoin can be given enterally (by various formulations) or parenterally. Phenytoin has the advantage of being able to be loaded rapidly to achieve steady-state concentrations in emergency situations. Historically, phenytoin was the most commonly used AED in SAH for many years. In studies that reported the use of phenytoin in SAH, the proportion of patients who received phenytoin ranged from 17 to 100%.Reference Choi, Chun, Yi, Ko, Kim and Kim 7 , Reference Murphy-Human, Welch, Zipfel, Diringer and Dhar 15 , Reference Baker, Prestigiacomo and Solomon 18 - Reference Karamchandani, Fletcher, Pandey and Rajajee 20 , Reference Chumnanvej, Dunn and Kim 25 , Reference Ibrahim, Fallah and Macdonald 26 , Reference Taylor, Heinrichs, Janzen and Ehtisham 28 , Reference Szaflarski, Sangha, Lindsell and Shutter 42 Though many studies have reported using phenytoin in patients, they did not elaborate on the effects of phenytoin on seizure frequency or further outcomes. In a study where 95% of the patients were treated with phenytoin and 67% had “therapeutic levels,” 4.5% experienced seizures.Reference Baker, Prestigiacomo and Solomon 18 The authors found no association between total duration of postoperative AED therapy and risk of late seizures, and they concluded that patients might benefit from therapeutic levels of anticonvulsant drugs in the immediate postoperative period following an SAH. Similarly, in another study where 88% of the study population received AED therapy “mostly” with phenytoin, 4.1% of the entire population experienced late seizures.Reference Butzkueven, Evans and Pitman 13
The duration of phenytoin therapy was studied by Chumnanvej et al.Reference Chumnanvej, Dunn and Kim 25 They compared patients treated with a phenytoin loading dose (LD) of 1000 mg followed by a maintenance dose (MD) of 100 mg three times daily from admission until discharge (as per the institutional protocol prior to 1999), to patients treated with a 3-day course (based on a change in the protocol in 1999). Serum phenytoin levels were not monitored, and so it is unclear if patients were receiving appropriately therapeutic doses. The incidence of seizures and mortality rate did not differ between the two groups; however, there was a significant reduction in occurrence of adverse effects in patients who received the 3-day course of phenytoin. This led the authors to conclude that a 3-day course of phenytoin “prophylaxis” is sufficient for patients with an SAH. Further, Naidech et al.Reference Naidech, Kreiter and Janjua 22 published findings that raised concerns about the use of phenytoin in patients with an SAH. Patients with SAH were appropriately loaded with phenytoin on admission and continued on maintenance doses of 5 mg/kg/day, and those with a higher “phenytoin burden” (defined as the patient’s average serum phenytoin level multiplied by the duration of phenytoin therapy to a maximum of 14 days) were found to be more likely to have poor functional and cognitive outcomes at 14 and 30 days post-SAH than those with a lower burden.
Many adverse effects attributed to phenytoin have been reported, ranging from 4 to 81%.Reference Lin, Chang and Chang 11 , Reference Murphy-Human, Welch, Zipfel, Diringer and Dhar 15 , Reference Rhoney, Tipps, Murry, Basham, Michael and Coplin 19 , Reference Shah and Husain 32 Reported adverse reactions in addition to difficulty maintaining therapeutic serum levels include difficulty optimizing cognitive recovery, rash, thrombocytopenia, anemia, abnormal liver function tests, ataxia, and fever.Reference Lin, Chang and Chang 11 , Reference Karamchandani, Fletcher, Pandey and Rajajee 20 , Reference Shah and Husain 32 When adverse effects were observed more frequently in patients treated with phenytoin compared to levetiracetam, a corresponding crossover rate to levetiracetam ranging from 40 to 66% has been reported.Reference Karamchandani, Fletcher, Pandey and Rajajee 20 , Reference Shah and Husain 32 These findings suggest that patients may tolerate levetiracetam better than phenytoin. Furthermore, clinicians need to be aware of the many significant drug/drug interactions involving phenytoin. Phenytoin is a strong inducer of CYP 2C and 3A, which are both responsible for the metabolism of many other medications. Such enzyme induction often results in a clinically significant decrease in serum levels of other medications, which may in turn warrant a dose increase or even contraindicate the use of one or another drug. Of importance in SAH is the interaction between phenytoin and nimodipine. Phenytoin induces the metabolism of nimodipine, which has been shown to result in an 86% reduction in nimodipine exposure.Reference Tartara, Galimberti and Manni 43 Although this finding has been reported in non-SAH patients on long-term phenytoin therapy, enzyme induction may occur as early as 4 days following initiation of phenytoin therapy, and that is within the highest risk period for DCI in most SAH patients.Reference Fleishaker, Pearson and Peters 44
Levetiracetam
The body of evidence for levetiracetam use in SAH is growing. Levetiracetam is available as oral and parenteral formulations and can be rapidly loaded. The parenteral formulation is not manufactured in Canada but can be obtained through the Health Canada Special Access Program. Levetiracetam displays linear elimination kinetics; therefore, dose changes produce relatively predictable changes in serum concentrations. In addition, levetiracetam has minimal drug/drug interactions.Reference Patsalos, Berry and Bourgeois 41
Several studies have compared the use of phenytoin and levetiracetam in patients with SAH.Reference Murphy-Human, Welch, Zipfel, Diringer and Dhar 15 , Reference Karamchandani, Fletcher, Pandey and Rajajee 20 , Reference Taylor, Heinrichs, Janzen and Ehtisham 28 , Reference Shah and Husain 32 , Reference Szaflarski, Sangha, Lindsell and Shutter 42 A trend toward similar or lower seizure incidence has been reported in levetiracetam-treated patients.Reference Karamchandani, Fletcher, Pandey and Rajajee 20 , Reference Taylor, Heinrichs, Janzen and Ehtisham 28 , Reference Shah and Husain 32 , Reference Szaflarski, Sangha, Lindsell and Shutter 42 On the other hand, Murphy-Human et al.Reference Murphy-Human, Welch, Zipfel, Diringer and Dhar 15 reported a higher rate of late seizures in levetiracetam-treated patients compared to phenytoin. However, the dose given was 500 mg twice daily, which may arguably be considered “subtherapeutic” in light of an analysis of levetiracetam elimination kinetics in neurocritical care patients (see the next section).Reference Spencer, Jacobi, Juenke, Fleck and Kays 45 The mild and tolerable side effects of levetiracetam have also been shown to favor its use over phenytoin. Several studies have shown a lower incidence of such adverse effects as gastrointestinal upset, worsened neurological status, elevated liver function tests, thrombocytopenia, and fever in patients treated with levetiracetam compared to phenytoin,Reference Shah and Husain 32 , Reference Szaflarski, Sangha, Lindsell and Shutter 42 as well as a high rate of crossover from phenytoin to levetiracetam.Reference Murphy-Human, Welch, Zipfel, Diringer and Dhar 15 , Reference Karamchandani, Fletcher, Pandey and Rajajee 20 , Reference Shah and Husain 32 Given the retrospective nature and low power of most of the studies, conflicting findings have been reported. While improved GOS scores and functional outcomes following discharge have been reported in patients treated with levetiracetam compared to phenytoin,Reference Taylor, Heinrichs, Janzen and Ehtisham 28 other researchers have found no difference in functional outcomes, hospital length of stay, and mortality.Reference Murphy-Human, Welch, Zipfel, Diringer and Dhar 15 , Reference Karamchandani, Fletcher, Pandey and Rajajee 20 , Reference Szaflarski, Sangha, Lindsell and Shutter 42
An appropriate duration of levetiracetam following an SAH has yet to be determined. A studyReference Murphy-Human, Welch, Zipfel, Diringer and Dhar 15 comparing a three-day course of levetiracetam to an extended course of phenytoin found a higher incidence of late seizures in the levetiracetam group. However, it is difficult to ascribe the difference in late seizure incidence to subtherapeutic dosing, inadequate duration of therapy, or possibly both. To follow up, a recent randomized open-label studyReference Murphy-Human, Diringer and Zipfel 46 presented as a conference abstract compared a three-day course of levetiracetam to an extended course in patients with an SAH. Interestingly, these results showed that the extended course of levetiracetam was associated with worse functional outcomes than the three-day course. Furthermore, a post-hoc analysis of a recent retrospective study reported that levetiracetam prophylaxis may be associated with a higher risk of delayed neurological deficits than no prophylaxis.Reference Panczykowski, Pease and Zhao 40 This contributes to the evidence suggesting that seizure prophylaxis may contribute to detrimental outcomes.
Valproic acid
Several studies have reported the use of valproic acid (VPA) in patients with an SAH.Reference Choi, Chun, Yi, Ko, Kim and Kim 7 , Reference Fung, Balmer and Murek 9 , Reference Mink, Muroi, Seule, Bjeljac and Keller 27 , Reference Yoon, Joo, Kim, Hong and Chung 37 , Reference Rejdak, Papuc, Dropko and Stelmasiak 47 In a small prospective trial comparing the use of VPA to levetiracetam in SAH,Reference Mink, Muroi, Seule, Bjeljac and Keller 27 no difference was observed in the incidence of seizures between the two groups.Reference Mink, Muroi, Seule, Bjeljac and Keller 27 However, 65% of patients on VPA were switched to another AED, whereas only 22% of patients on levetiracetam required switching. The reasons for switching included having plasma concentrations below the reference range, occurrence of seizures, elevated liver function tests, or evidence of clinical coagulopathy. Another study,Reference Choi, Chun, Yi, Ko, Kim and Kim 7 where 72% of AED-treated patients received VPA, reported an incidence of adverse effects of 23% among the AED-treated patients (e.g., rash, fever, dizziness, thrombocytopenia, hepatitis, hypotension, and vasospasm). Based on very limited evidence, VPA appears to be associated with a higher incidence of adverse effects than levetiracetam. If further use of VPA in SAH is to be considered, then prospective research comparing its efficacy and safety to other AEDs (such as phenytoin or levetiracetam) would be warranted. However, VPA could be useful as a second-line agent to control refractory seizures.Reference Rejdak, Papuc, Dropko and Stelmasiak 47
In conclusion, phenytoin has been used most commonly in SAH, though its use has been linked to poor long-term cognitive outcomes and increased rates of adverse drug reactions. Studies comparing the use of levetiracetam and phenytoin in SAH have reported mixed findings regarding seizure occurrence, but many have suggested that levetiracetam is better tolerated than phenytoin.
Dosing and Monitoring Antiepileptic Drugs in SAH
Phenytoin Dosing and Monitoring
Kim et al.Reference Kim, Kim, Ji and Chun 48 explored the pharmacokinetics of fosphenytoin loading in patients with SAH. They found that total phenytoin serum concentrations remained above the reference range upper limit of 80 µmol/L in 28/30 patients for the first 20 minutes following infusion of a 20-mg/kg dose over 150 mg/min. The mean serum concentration at 24 hours following the loading dose was 48 µmol/L (reference range=40–80 µmol/L). Although 4 patients experienced a drop in mean blood pressure greater than 20 mmHg, none experienced serious adverse reactions.Reference Kim, Kim, Ji and Chun 48 Similarly, Rhoney et al.Reference Rhoney, Tipps, Murry, Basham, Michael and Coplin 19 reported that, following administration of a mean loading dose of 13.1 mg/kg, 20% of patients had supratherapeutic phenytoin levels (>80 µmol/L), 50% were within the reference range, and 30% were subtherapeutic (<40 µmol/L). Subtherapeutic phenytoin levels were not associated with in-hospital seizures. In addition, patients who received a loading dose were less likely to have seizures than those who did not. Based on that, regular phenytoin initial dosing is suggested in SAH patients.
Phenytoin displays nonlinear Michaelis–Menten kinetics, meaning that the rate of elimination is dose-dependent: as the dose increases, the rate of phenytoin metabolism becomes saturated and the elimination half-life increases.Reference Patsalos, Berry and Bourgeois 41 As a result, small dose changes yield disproportionate and unexpected changes in serum concentrations. This, combined with large interindividual variability, warrants meticulous monitoring of serum phenytoin levels in addition to clinical/EEG monitoring of patients.
Levetiracetam dosing and monitoring
Augmented renal clearance (ARC), defined as a creatinine clearance (CrCl) exceeding 130 ml/min, has been reported in patients with an SAH, given the young age at onset.Reference Drust, Luchtmann, Firsching, Troger, Martens-Lobenhoffer and Bode-Boger 49 , Reference May, Arora, Parli, Fraser, Bastin and Cook 50 Levetiracetam renal clearance has been reported to be directly related to creatinine clearance in SAH, resulting in a need for higher than regular doses to achieve seizure control in patients with ARC.Reference Spencer, Jacobi, Juenke, Fleck and Kays 45 A Monte Carlo simulation conducted by Spencer et al.Reference Spencer, Jacobi, Juenke, Fleck and Kays 45 based on pharmacokinetic data obtained from 12 patients (10 of whom had an SAH) showed that the regimen most likely to achieve serum levetiracetam levels within the suggested reference range (6–20 μg/mL) was 1000 mg IV Q8H. In addition, the regimen least likely to achieve levels within the reference range was 500 mg IV Q12H. Furthermore, Drust et al.Reference Drust, Luchtmann, Firsching, Troger, Martens-Lobenhoffer and Bode-Boger 49 reported an SAH patient who required a total levetiracetam daily dose of 4 g to maintain therapeutic levels and remain seizure-free. This has been attributed to the patient’s ARC (CrCl ~ 160 mL/min).
In addition to enhanced levetiracetam clearance, it seems that the oral bioavailability of levetiracetam may also be reduced, further affecting drug exposure. It has been reported that switching intravenous levetiracetam to an oral liquid in SAH patients resulted in a mean decline of 30% in levetiracetam plasma concentrations.Reference Mink, Muroi, Seule, Bjeljac and Keller 27 The authors hypothesized that critically ill patients may have altered absorption of the medication, suggesting that clinicians might consider increasing the dose of levetiracetam by 30% when converting from parenteral to oral liquid dosing.Reference Mink, Muroi, Seule, Bjeljac and Keller 27 Further evaluation of the pharmacokinetics of levetiracetam in patients with an SAH is needed. Until then, clinicians choosing to use levetiracetam in this setting may consider an initial regimen of 1000 mg IV/PO Q8–12.Reference Spencer, Jacobi, Juenke, Fleck and Kays 45
Monitoring levetiracetam concentrations is generally unnecessary given its linear and predictable pharmacokinetics and minimal drug interactions. However, given the altered pharmacokinetics in the SAH population, serum levetiracetam level may be of value, especially in patients with refractory seizures. In addition to clinical/EEG monitoring, close monitoring of renal function in patients on levetiracetam is essential given its renal elimination. The adverse effects of levetiracetam are minimal, with sedation being the most common—and usually transient. The more rare psychiatric and behavioral side effects should serve as a precaution for use in patients with preexisting psychiatric comorbidities.Reference Perucca and Gilliam 51
Conclusions
The current evidence is largely derived from retrospective and observational data and thus has limited validity due to unaccounted-for biases and confounders. However, clinicians are left with the available evidence on which to base their decisions. Given the reported adverse outcomes associated with AED use, seizure prophylaxis is not warranted; however, it might need to be considered in SAH patients with risk factors for seizure recurrence. The duration of AED therapy remains a point of debate, but it seems that continuation of AEDs post-discharge is not strongly supported unless the patient develops post-SAH epilepsy. Levetiracetam appears to be better tolerated than phenytoin in SAH patients. Ideally, a randomized controlled trial comparing the use of phenytoin, levetiracetam, and placebo in SAH powered to detect a meaningful difference in seizures and adverse effects would provide a more clear direction for deciding which AED is best to use in patients with SAH. Also, the utility of other AEDs in SAH has not as yet been explored. In addition, further studies are needed to characterize and confirm levetiracetam pharmacokinetics in SAH patients.
Disclosures
The authors do not have anything to disclose.




