Richter’s syndrome (RS) is defined as the transformation of a B-cell chronic lymphocytic leukemia (CLL) into an aggressive lymphoma. The diffuse large B-cell lymphoma (DLBCL) is the most common type in RS, occurring in 5%–10% of CLL patients. Median time to RS transformation is 23 months. The majority of cases of RS involves lymph nodes, being the extra-nodal involvement rare.
Regarding the central nervous system (CNS) involvement in RS, isolated parenchymal lesions in the brain occur in few cases.
In general, RS is a rapidly progressive disease and usually chemotherapy resistant; a median survival time of 8 months and 5-year survival rate <5% are reported. Reference Rossi, Cerri and Capello1,Reference Parikh, Rabe and Call2
Here, we present a patient who developed an isolated CNS-RS. We examined the clonal relationships of the CNS-RS lesion with the original CLL cells and investigated MyD88 mutation.
A 74-year-old male was seen in 2009 because of leukocytosis and diagnosed with CLL, Binet stage A. Because of the absence of an active disease, a watch and wait strategy was performed. In March 2013, the patient progressed to a Binet stage C. The bone marrow biopsy showed evidence of a diffuse infiltration of B-CLL (85%) with deletion of region 13q14 in 50% of nuclei at the FISH analysis, and the IgHV gene had two productive rearrangements >2% (mutated CLL). The patient received chemotherapy with Chlorambucil and Rituximab, achieving an objective response.
On March 2015, the patient relapsed and received chemotherapy with Bendamustine and Rituximab and a bone marrow’s biopsy showed a reduction of infiltration of B-CLL (<5%) following treatment. At this point, the patient started a close follow-up.
Three years later, the patient developed a sudden cognitive impairment, fatigue, and evidence of right motor hemiparesis. A magnetic resonance of the brain (MRI) showed an intense and homogenous enhancing lesion in the left cerebellar peduncle with an extension to left thalamus and internal capsule (Figure 1), suspicious for either a primary CNS lymphoma (PCNSL) or a CNS localization of CLL (CNS-RS).
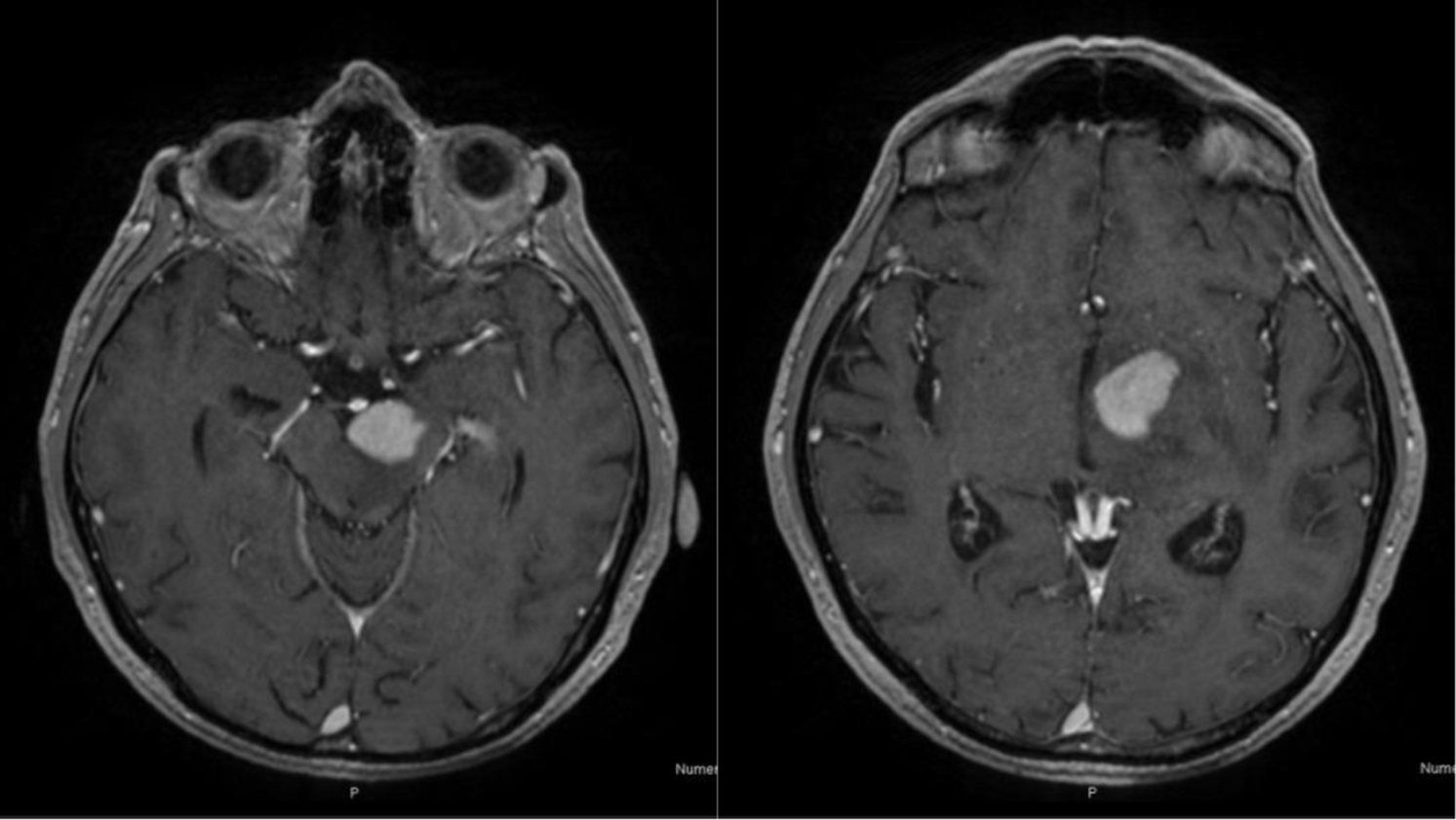
Figure 1: Axial T1-weighted images showing an intense and homogeneous enhancing lesion in the left cerebral peduncle extended to left thalamus and internal capsule.
The 18F-FDG positron emission tomography showed no evidence of systemic disease.
Cerebrospinal fluid analysis revealed the presence of atypical lymphocytic elements of medium-large size, highly suspicious for a lymphoproliferative disease. There was also evidence of mutation of MyD88 in the CSF and the peripheral blood. A stereotactic biopsy was performed and showed an aggressive large B-cell lymphoma CD5+, CD20+, CD79a+, CD3−, CD23−, CD10−, BCL6+, and BCL2+. Tumor sample was positive for MyD88. The PCR analysis of IgH clonality showed a monoclonality and the same rearrangement pattern of the previous analysis of 2015.
A diagnosis of RS was made, and given the poor performance status (KPS 50) and advanced age, a regimen with high-dose Methotrexate (due to the activity in the CNS) and Rituximab was started.
After II cycles of chemotherapy, the clinical condition worsened. The brain MRI showed a volumetric increase of the lesion, suggestive of a progression of the disease.
The therapy was interrupted, and the patient passed away 3 weeks later.
The CNS-RS is rare, and we found 18 cases in the literature since 1988 (Table 1).
Table 1: Reported cases of Richter’s syndrome localized in the central nervous system (CNS)

CLL = chronic lymphocytic leukemia, NA = not available, RS = Richter’s syndrome, MTX = Methotrexate, RTX = Rituximab, RT = Radiotherapy, CT = Chemotherapy.
* Correspondance between the rearrangement of IgH genes in CLL and the CNS-RS.
The median age is 65 years, and median time to transformation for CNS-RS was 36 months, with the longest duration being 10 years from CLL diagnosis (as in our patient). Conversely in 3 patients, the CNS-RS diagnosis was concurrent with the CLL diagnosis.
RS presents as DLBCL with an ABC phenotype in almost 90% of cases, and the clinical progression of CLL is usually associated with an increase in size and proliferative capacity of the cells. In RS, tumor cells express CD20, while CD5 expression is maintained only in a fraction of cases (almost 30%). Several studies have identified baseline clinical and biological characteristics of CLL associated with an increased risk of RS: advanced Binet Stage, bulky adenopathy, CD38, CD49d, and Zap-70 expression, unmutated IGHV, del11q, and del17p. Reference Parikh, Rabe and Call2,Reference Condoluci and Rossi3,Reference Rossi4 In our patient, except for the advanced Binet stage, we did not find other risk factors of RS.
Based on the analysis of the rearrangement of IGHV-D-J genes, 80% of the DLBCL variants of RS are clonally related to the preceding CLL, representing a real transformation. In other cases of RS, there are distinct IGHV-D-J rearrangements compared to the preceding CLL, representing de novo lymphomas. Reference Mao, Quintanilla-Martinez and Raffeld5 The type of clonal pattern has an impact upon prognosis, with clonally related cases having a median survival of around 12 months, whereas clonally unrelated RS has a median survival of 65 months. Reference Rossi, Spina and Deambrogi6
In seven cases of CNS-RS reported in literature, the analysis of the rearrangement of IGHV-D-J genes related to the preceding CLL was performed and showed a correspondence. In the other 11 cases, the analysis was not available.
To our knowledge, this is the first case in whom the MyD88 mutation in a CNS-RS was investigated and found positive in the peripheral blood, CSF, and tissue sample. According to the literature, MYD88 L265P mutation is a significant mutation in DLBCL Reference Lee, Jeong, Choi, Oh and Kim7 and is very rare in CLL without RS. Thus, testing of MyD88 mutation in CSF in patients with suspicion of RS should be performed.
Therapeutic options for primary CNS lymphomas in CNS-RS have included MTX combined with rituximab and sometimes salvage or adjuvant radiotherapy. Survival ranged from 10 days to 2 years, and we observed a median survival time of 4 months, shorter than the median survival time of 8 months for RS not involving the CNS. Reference Mao, Quintanilla-Martinez and Raffeld5,Reference Rossi, Spina and Deambrogi6
RS of the CNS is a very rare and aggressive entity. The diagnosis is difficult, especially when developing many years after the CLL diagnosis. To achieve a diagnosis of isolated CNS-RS, a complete pathological and molecular study is needed in order to confirm the suspicion of a CNS localization of CLL and the analysis of the rearrangement of IGHV-D-J genes is critical.
An optimal treatment is not available. Therapeutic regimens for PCNSL have been used in most cases of CNS-RS; unfortunately, the results have been generally disappointing due to chemotherapy resistance and poor performance status of the patients. The MyD88 mutation could be an option for future target therapies.
Disclosures
The authors have no conflicts of interest to declare.
Statement of Authorship
EP, FM, DG, RP, RR, and RS followed the patient and write the article. LB, ML, and MFF help us with the diagnosis and the writing process of the article.




