Progressive supranuclear palsy (PSP) is an atypical parkinsonian syndrome comprising of supranuclear vertical gaze palsy, pseudobulbar affect, postural instability, along with cognitive decline. Progressive supranuclear palsy is a neurodegenerative tauopathy. However, there are several conditions that can clinically mimic PSP presentation. New diagnostic criteria were developed by the International Parkinson and Movement Disorders Society (MDS)Reference Hoglinger, Respondek and Stamelou1 to improve the sensitivity and specificity for diagnosis of PSP/PSP variants. We report a case of an atypical PSP and emphasize the approach to PSP and other PSP-like presentations.
A 75-year-old right-handed male presented with a 5-year-history of slow progressive parkinsonism with gait difficulty and falls in the absence of tremor. He also had slow progressive cognitive decline over the course of 3 years with impaired episodic memory and executive function. He did not have urinary incontinence or behavioral changes. He took 1000 mg of levodopa daily for 2 years with poor response. He did not report dyskinesia.
Examination showed normal blood pressure and pulse (120/76 mmHg and 88 per minute, supine) with no postural changes (120/72 mmHg and heart rate 92 per minute, standing). He scored 19/30 on the Montreal Cognitive Assessment, with difficulties in visuospatial tasks, attention, verbal fluency and delayed recall (improved with categorical cues). He had ideomotor apraxia with impaired spatiotemporal aspects of movements, but did not have any cortical sensory loss or alien limb phenomenon. He had restricted vertical gaze and horizontal gaze to the right only, saccadic pursuits, and impaired saccadic initiation and velocity in the absence of square wave jerks. His gaze restrictions were overcome by the vestibulo–ocular reflex. On optokinetic nystagmus (OKN) testing, there was preserved corrective nystagmus to the right when the OKN strip moved to the left, but OKN was impaired when the OKN strip moved to the right and vertically. He was dysarthric with palilalia (online Supplementary Video 1). He had axial and appendicular rigidity (left > right). Power was intact. There was bradykinesia with finger tapping and rapid alternating movements, with frequent arrests of movements. There was subtle asymmetry with deep tendon reflex (DTR). It was easier to elicit his DTRs at the right side, but they were still 2+. He had flexor plantar response bilaterally. There were no other pyramidal findings. There were no frontal release signs on examination. Applause sign was negative. Gait was slow with decreased stride length and reduced arm swing bilaterally. He had poor postural reflexes on pull testing (online Supplementary Video 1). Through the course of follow-up, his parkinsonism and balance progressed, along with his cognitive function. However, the extraocular movements did not evolve from the initial examination.
Restricted vertical gaze, with asymmetrical restriction with horizontal gaze (only to the right) is not characteristic of degenerative tauopathies. The brain MRI performed 2 years from onset of symptoms demonstrated multiple cortical bleeds, and a right pontine bleed, which explained his asymmetrical horizontal gaze restriction. Basal ganglia microbleeds were also evident (Figure 1). There were multiple ischemic changes in the midbrain and frontal white matter bilaterally. These changes can be seen in association with hypertension, aging and cerebral amyloid angiopathy (CAA). This case is atypical for degenerative PSP given the normal midbrain size and midbrain to pons ratio (Figure 1)Reference Massey, Jager and Paviour2 and the other atypical clinical and radiological findings. A repeat MRI 5 years after the initial scan showed progressive brain atrophy. Midbrain measurement and midbrain to pons ratio remained normal. Such findings argue against PSP. There was an increase in microbleeds in the subcortical regions, which might explain the slow cognitive decline.
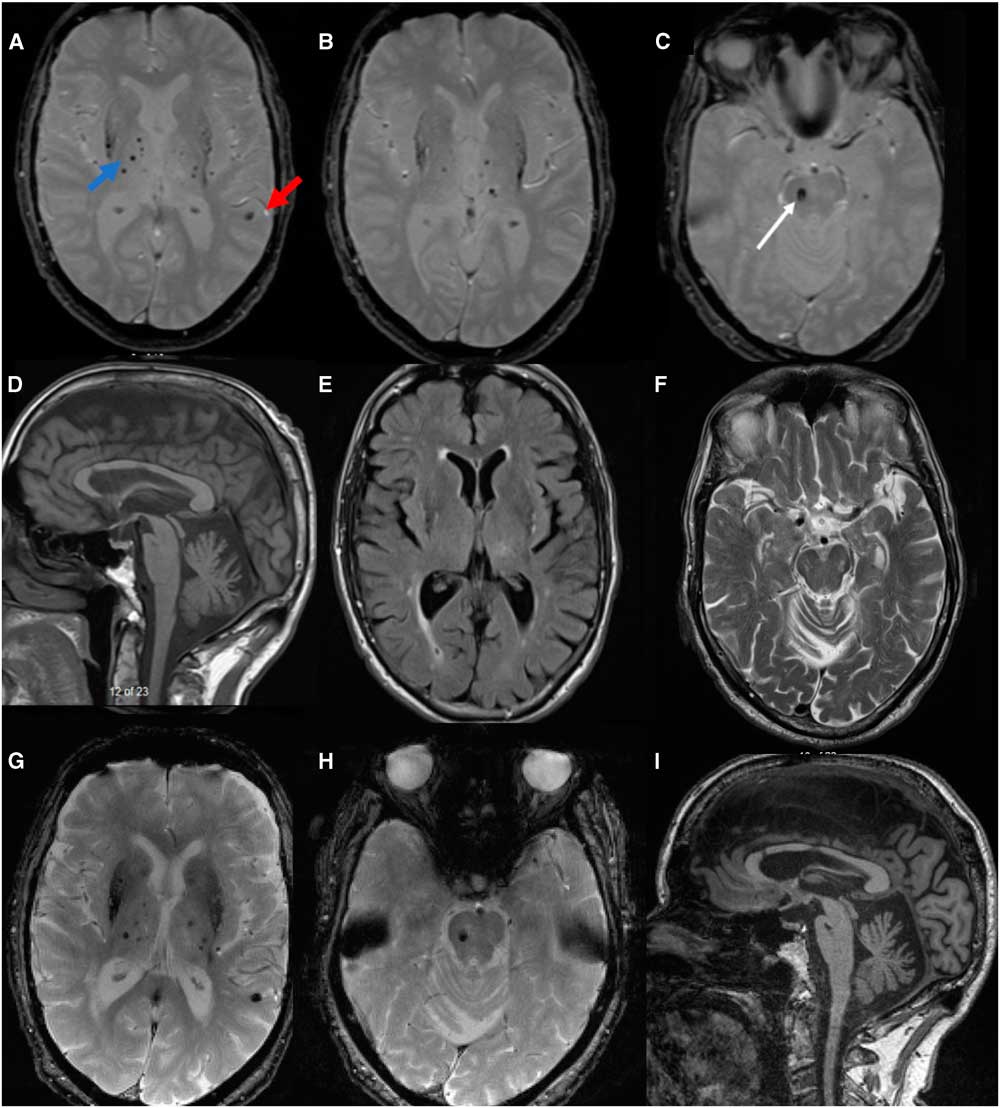
Figure 1 MRI brain (A and B): axial gradient echo demonstrating multiple areas of signal drop out best seen as blooming in the cortical (red arrow) and subcortical region involving bilateral putamen (blue arrow) and surrounding subcortical structures, including thalami suggestive of multiple bleeds; (C): at the level of pons, the white arrow points to an area of signal drop out at the right pons, suggestive of right pontine bleed. This correlates with the clinical finding of right gaze restriction. The structure of the dorsal midbrain, basal ganglia and thalamus are shown in D, E and F. The ratio of midbrain to pons in T1W mid-sagittal section is 0.69, which argue against PSP (D).Reference Massey, Jager and Paviour2 Axial midbrain T2 sequence showed no atrophy, and dorsal midbrain is measured at 10 mm. With repeat MRI 5 years later (G-I), no significant worsening was seen on the T2* sequence at the level of pons and basal ganglia when compared with the initial MRI. There is no progressive dorsal midbrain atrophy (I). However, progressive cerebral atrophy was seen. PSP = progressive supranuclear palsy.
Conditions like multiple system atrophy (MSA),Reference Anderson, Luxon, Quinn, Daniel, David Marsden and Bronstein3 dementia with lewy bodies (DLB),Reference Clerici, Ratti and Pomati4 Alzheimer’s disease, motor neuron disease and vascular etiologies can present with parkinsonism, supranuclear gaze palsy and postural instability. Based on the MDS PSP diagnostic criteria,Reference Hoglinger, Respondek and Stamelou1 the exclusion criteria require these conditions to be ruled out on history, clinical examination or imaging. While MSA or DLB are to be chiefly excluded on clinical grounds, the criteria also lay out several conditions that need to be excluded based on their characteristic MRI findings. These include severe leukoencephalopathy, relevant structural abnormalities, vascular parkinsonism along with its associated syndromes (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy and CAA) and prion disease.
Among the PSP mimics, DLB, spinocerebellar ataxias, MSA and frontotemporal dementia with chromosome 17 mutations can exhibit parkinsonism, postural instability and supranuclear gaze palsy (Table 1). Our patient did not have visual hallucinations, fluctuating sensorium, rapid eye movement sleep behavioral disorder, autonomic dysfunction, ataxia or behavioral issues making the above diagnoses unlikely. He had no clinical history evidence for ischemic stroke, making typical vascular parkinsonism unlikely. He did not have oculomasticatory myorhythmia, although he had ocular findings that could still suggest Whipple disease; however, the MRI findings suggested an alternative diagnosis.
Table 1 List of the differential diagnoses of abnormal eye movements with postural instability and Parkinsonism [modified and updated from Williams and LeesReference Williams and Lees13]
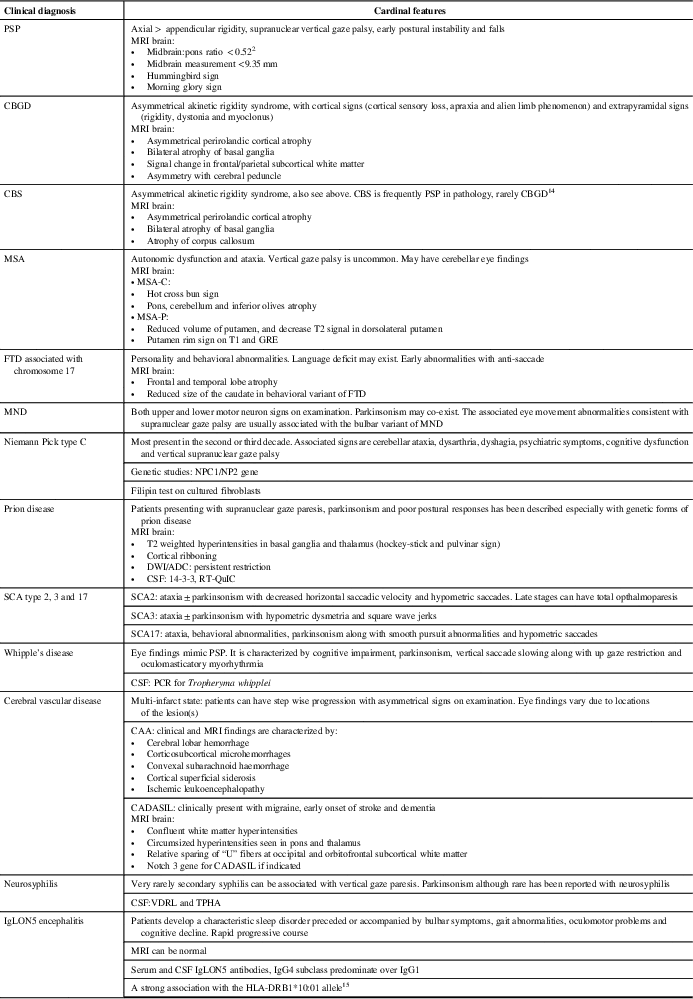
ADC = apparent diffusion coefficient; CAA = cerebral amyloid angiopathy; CADASIL = cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; CBGD = corticobasal degeneration; CBS = corticobasal syndrome; DWI = diffusion weighted imaging; FTD = frontotemporal degeneration; GRE = gradient echo; MND = motor neuron disease; MSA = multiple system atrophy; MSA-C = multiple system atrophy with cerebellar features; MSA-P = multiple system atrophy with parkinsonism; NPC1/NPC2 = NPC intracellular cholesterol transporter 1 = NPC = intracellular cholesterol transporter 2; PCR = polymerase chain reaction; PSP = progressive supranuclear palsy; SCA = spinocerebellar ataxia; TPHA = treponema pallidum haemagglutination test; VDRL = the venereal disease research laboratory.
The most common etiology for the cerebral microbleeds is CAA, which is classically associated with cortical bleeds. Sveinbjornsdottir et alReference Sveinbjornsdottir, Sigurdsson and Aspelund5 and Fisher et alReference Fisher, French, Ji and Kim6 reported that microhemorrhages were found in non-hypertensive subjects with no prior history of stroke, and concluded that microbleeds can occur in an aging brain with the most common site being the putamen irrespective of hypertension status. The most common pathology found in these patients was CAA, while atherosclerosis with fibrinohyalinosis or co-existing dual pathologies are less common.
Cerebral amyloid angiopathy is a heterogeneous disorder with amyloid deposition in the small to medium sized leptomeningeal and cortical arterioles. Clinically, CAA associated vasculopathy is characterized by lobar cerebral hemorrhages, cortical micro-hemorrhages, superficial siderosis, subarachnoid hemorrhage and cortical infarcts with ischemic white matter changes. Very rarely, it can present with an encephalopathy in CAA related angiitis.Reference Biffi and Greenberg7 Interestingly, some pathological studies have shown a higher incidence of CAA deposition in idiopathic Parkinson’s disease (53% vs. 24% in control), more pronounced in patients with DLB and Parkinson dementia complex.Reference Jellinger and Attems8 A multi-infarct state may also present with a PSP spectrum presentation (CT, MRI brain or autopsy).Reference Dubinsky and Jankovic9
This case presents an atypical PSP-like syndrome with cerebral microbleeds secondary to CAA being the most likely etiology. To date, only one previous series demonstrated a single patient with pathological evidence of CAA. The reported case had vascular lesions secondary to CAA in the right centrum semiovale, substantia nigra, diencephalon and pons.Reference Dubinsky and Jankovic9 Another case series by Josephs et alReference Josephs, Ishizawa, Tsuboi, Cookson and Dickson10 demonstrated four pathological cases with vascular lesions presenting as PSP-like syndrome in the absence of tauopathy. The patients may have asymmetrical neurological signs such as cranial nerve VII palsy, hemiparesis, the Babinski sign or leg dystonia which could serve as clinical clues to differentiate from PSP. Interestingly, all subjects had frontal infarcts, three had unilateral basal ganglia and right thalamic infarcts, while bilateral thalamic infarcts were seen in one patient. Similarly, only one patient had infarcts in the basis pontis and medullary tegmentum. In our patient with multiple microbleeds, while CAA or atherosclerosis with fibrinohyalinosis is the likely pathology, whether he has co-existing dual pathologies remains unproven without tissue diagnosis or tau imaging.
Classically, structural lesions involving the rostral interstitial nucleus of the medial longitudinal fasciculus, the interstitial nucleus of Cajal, and the posterior commissure will cause vertical gaze palsy, but that was not the case with our patient. Supranuclear vertical gaze palsy, in our case, may be secondary to the midbrain stroke or disruption by the thalamomesencephalic lesions, both of which have been described previously to cause vertical gaze restrictions.Reference Iijima, Hirata, Tadano, Kamakura and Nagata11, Reference Clark and Albers12 Similarly, the parkinsonism and poor postural balance are likely secondary to the disruption of the cortico-pallidal-nigro-thalamo-cortical loops.
Although a co-associated tauopathy cannot be ruled out, it remains imperative to exclude PSP mimics by following the MDS diagnostic criteria before designating PSP as the final diagnosis. Many of these PSP-mimics, such as Niemann Pick type C, neurosyphilis, IgLON5 encephalitis and whipple disease may be treatable and carry different prognosis than PSP itself. Careful history and examination with appropriate neuroimaging (Figure 2), including gradient echo or susceptibility weighted imaging, are required before making the diagnosis of PSP.

Figure 2 Flowchart for approach to a patient with parkinsonism, supranuclear gaze palsy and postural instability. PSP = progressive supranuclear palsy; CBS-PSPS = corticobasal syndrome-progressive supranuclear palsy syndrome; MSA = multiple system atrophy; MSA-C = multiple system atrophy with cerebellar features; MSA-P = multiple system atrophy with parkinsonism; CAA = cerebral amyloid angiopathy; CADASIL = cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; FTD = frontotemporal degeneration; CJD = Creutzfeldt–Jakob disease; VDRL = venereal disease research laboratory; TPHA = treponema pallidum haemagglutination test; NPC1/NPC2 = NPC intracellular cholesterol transporter 1/NPC intracellular cholesterol transporter 2; PCR = polymerase chain reaction.
Funding Sources
There are no funding sources for this case report. Repeat imaging was performed as part of the Function Assessment of Vascular Reactivity Study (Eric E. Smith, PI, University of Calgary, Richard Camicioli, site-principal investigator, University of Alberta, funded by Brain Canada and Canadian Institutes of Health Research).
Authors’ Contributions
AS participated in patient evaluation, drafting/revising the manuscript and acquisition of data. RC participated in study concept and critical revision of the manuscript. FB participated in study concept, acquisition of data and critical revision of the manuscript.
Conflicts of Interest/Financial Disclosures
The authors declare that they have no competing interests. The authors report no financial disclosures. FB, AS and RC have nothing to disclose.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2018.367





