Introduction
Parkinson’s disease (PD) is a chronic, progressive neurodegenerative disease. The cardinal motor symptoms of PD are tremor at rest, rigidity, bradykinesia, and postural instability. An array of non-motor symptoms (NMS) complicates the clinical course of most of the patients. Reference Kalia and Lang1 The estimated prevalence of PD in developed countries is 0.3% of the general population; however, the prevalence increases with age (>1% in people >60 years, >3% in people >80 years). Reference de Lau and Breteler2 The elderly population is not only burdened with a higher PD prevalence but also with several age-related comorbidities necessitating surgeries. Reference Etzioni, Liu, O’Connell, Maggard and Ko3 The surgeries may be elective or emergent and may be directly (deep brain stimulation (DBS)) or indirectly (for treatment of injuries related to recurrent falls) related to PD or may be unrelated as well (cataract surgeries, gastrointestinal surgeries, joint replacement surgeries, onco-surgeries, etc.). With the expansion of knowledge on NMS, PD is no longer considered a simple motor disorder of basal ganglia pathology, rather it is now being recognized as a clinically heterogenous multisystem disorder. Reference Klingelhoefer and Reichmann4 The existence of a large constellation of motor and NMS (Table 1) in elderly patients who are on several medications, often not limited dopaminergic agents, makes the management of the perioperative phase challenging. A population-based study in Taiwan revealed that patients with PD undergoing non-neurological surgeries have higher 30-day mortality and have a greater risk of having postoperative major complications including pulmonary embolism, stroke, pneumonia, septicemia, acute renal failure, and urinary tract infection (UTI). Reference Huang, Chou and Yeh5 Considering the challenges involved in the management of PD patients in the perioperative phase, it is crucial for the neurologists, anesthesiologists, and surgeons to be aware of the risks and complications to prevent and manage them effectively.
Table 1. Summary of commonly observed non-motor symptoms in Parkinson’s disease, perioperative risk factors, and prevention

The literature on perioperative management of PD is sparse. Moreover, substantial advances in PD therapeutics warrant updates in the knowledge about perioperative management of PD. This article revisits the important perioperative issues in PD and comprehensively describes the pragmatic approach to their management, taking into account the recent advances in the field of PD.
Preoperative Considerations
Accurate Reconciliation and Timely Administration of Home Medications
Patients with PD are advised to have their anti-Parkinson medications at specific times of the day or fixed intervals daily. Deviations in the schedule of such medications may result in bothersome adverse effects such as motor fluctuation and that, in turn, can be associated with fluctuations in the NMS of PD. Reference Kim, Kim and Shin6,Reference Martínez-Fernández, Schmitt, Martinez-Martin and Krack7 However, erroneous reconciliation (dose/route/frequency) of these time-sensitive medications is not uncommon for hospitalized PD patients. A large retrospective study from Spain (1628 patients, 2546 admissions) revealed that medication errors during hospitalization (in one-third of the admissions and half of the patients) were associated with higher mortality in PD. Reference Lertxundi, Isla and Solinís8 Several other studies have documented medication error rates ranging from 23% to 76% in hospitalized PD patients. Reference Derry, Shah, Caie and Counsell9–Reference Lance, Travers and Bourke11 The commonly documented errors include omission and/or incorrect scheduling of the dopaminergic agents, and administration of medications with relative contraindications in PD (e.g. older antipsychotics, antiemetics with dopamine-blocking property, and anticholinergics).
The reconciliation errors are probably higher for emergency hospitalizations, especially when the patients are on several medications that are non-formulary in the inpatient pharmacy. If patients have the non-formulary medications with them during the admission, the inpatient pharmacy verifies and lists the medications as “patient’s own medication”. Hence, patients may miss a dose or get an incorrect dose if the pharmacy delays the verification or does an inaccurate documentation of the medications and their doses. However, as mentioned above, patients are unlikely to carry their home medications during emergency hospitalization, which leads to incomplete medication reconciliation. The seemingly minor deviations in the anti-Parkinson treatment regimen may culminate in major issues directly (severe OFF-state, worsening of dyskinesias, dysphagia) or indirectly associated with PD (aspiration pneumonia, delirium, and falls). Reference Derry, Shah, Caie and Counsell9,Reference Magdalinou, Martin and Kessel10 Hence, strict adherence to PD patients’ individualized medication regimen is crucial. Education of the staff and effective implementation of PD-specific admission protocols may avoid complications during the perioperative phase and improve the outcomes of hospitalized PD patients. Reference Lance, Travers and Bourke11–Reference Aslam, Simpson, Baugh and Shill13 The Parkinson’s Foundation recommends using the “Aware in Care” kit, which helps in planning a safe hospitalization. 14
Addressing the NMS During the Hospital Stay
A plethora of NMS complicates the clinical course of PD by potentially affecting all the physiological systems. Certain NMS such as sleep disturbances, psychosis, pain, sialorrhea, constipation, and those resulting from dysautonomia (orthostatic symptoms, bladder disturbances) can be bothersome during the perioperative state. It is important in the preoperative state to document the NMS using the appropriate screening instruments. Reference Martinez-Martin, Chaudhuri and Rojo-Abuin15 Comprehensive documentation of the NMS before the procedures not only helps in distinguishing the NMS related to PD from those secondary to the procedures (delirium, urinary retention, pain, etc.) but also for effective management of such symptoms. Table 1 succinctly summarizes the NMS in PD and their perioperative implications. Comprehensive management of the NMS in PD is beyond the scope of this review and it is described in detail elsewhere. Reference Seppi, Ray Chaudhuri and Coelho16
Managing Parkinsonian Symptoms During NPO Status
One of the common prerequisites of all the surgeries under anesthesia is “nothing through mouth” (Latin – nil per os or NPO) status; however, oral medications may be taken by mouth with a sip of water almost up to the time of surgery in most cases and a strict NPO order is usually reserved for special circumstances (e.g. major gastrointestinal surgeries). However, several factors including the NPO status, knowledge gap of healthcare providers, and delays related to the hospital delivery system may result in prolonged periods of medication withholding. A retrospective analysis of 89 separate surgical events for 67 discrete PD patients revealed a median withholding duration of 12.35 h for carbidopa-levodopa (C/L). Reference Fagerlund, Anderson and Gurvich17 The median withholding duration was longer for inpatient procedures (16.75 h) than for the outpatient procedures (11.38 h). In the same study, 62% of the surgeries resulted in medication withholding duration between 10 and 20 h and 17% of the surgeries resulted in withholding times of >20 h. Reference Fagerlund, Anderson and Gurvich17
The physician should consider allowing the administration of PD medications as close as possible to the patient’s medication schedule preoperatively. Scheduled surgery should ideally happen early in the day to promote the best symptom management and limit the risk of complications and discomfort related to missed doses. Keeping the PD patients under strict NPO status is challenging because almost all the major anti-Parkinsonian medications are available only with oral formulations. Involving a Movement Disorders Neurologists or Neurologists with knowledge in PD management is recommended to help with change in medication regimen. As dopamine withdrawal syndrome can be devastating, Reference Rabinak and Nirenberg18 it is essential to be aware of alternative therapeutic options. Among the currently available anti-Parkinsonian medications, orally disintegrating C/L and non-oral formulations of levodopa or dopamine agonists such as rotigotine and apomorphine (sublingual/subcutaneous injections/pump) can be considered. Table 2 summarizes the dose conversions, advantages, and disadvantages of these non-oral alternatives of levodopa.
Table 2. Alternatives to oral levodopa/carbidopa in the perioperative setting in Parkinson’s disease

AE = Adverse effects; C/L = carbidopa/levodopa; CLES = carbidopa-levodopa enteral suspension; GI = gastrointestinal; LED = Levodopa equivalent dose; NPO = Nil per os; OH = orthostatic hypotension; SC = subcutaneous.
Intraoperative Considerations
Challenges in Positioning and Monitoring
Problems with proper positioning may arise in patients with inadequately managed Parkinsonian symptoms in the setting of regional anesthesia. While general anesthesia would temporarily suppress the Parkinsonian symptoms, those under regional anesthesia may have persistence of rigidity in the neck or in the extremities and tremor of several parts of the body. Such scenarios reinforce the importance of optimal dopamine replacement therapy in the preoperative phase as described above. Infrequently, patients may have other PD-associated musculoskeletal deformities such as camptocormia, striatal hand, and foot deformities which would potentially make proper positioning of patients challenging. Reference Wijemanne and Jankovic19 Hence, depending on the site of surgery, proper planning regarding the type of anesthesia and positioning should be done in advance.
Monitoring of several physiological parameters such as temperature, blood pressure, and cardiac rhythm may be affected by a number of PD-associated factors. For example, thermoregulatory dysfunction is common in PD, resulting in heat and cold intolerance. Although rare, the anesthesiologists should be mindful of the possibilities of spontaneous periodic hypothermia in PD patients which can have certain EKG and EEG correlates. Reference Li and Lou20–Reference Coon and Low22 Similarly, hyperthermia can be a manifestation of the Parkinsonism-hyperpyrexia syndrome which usually results from the abrupt cessation of the dopaminergic agents. Reference Newman, Grosset and Kennedy23 Hence, abrupt changes in temperature should be closely monitored in the intraoperative phase. In patients undergoing regional anesthesia, the presence of tremors can result in artifacts in the EKG which mimic atrial flutter or ventricular fibrillation. In addition, excessive sweating due to dysautonomia may result in poor EKG electrode contact with the skin. Hence, an abrupt change in cardiac rhythm should prompt a look into these common artifacts.
Addressing the Common Respiratory and Cardiovascular Complications
Several studies have reported suboptimal respiratory function in PD patients. Reference Hovestadt, Bogaard, Meerwaldt, Van der Meche and Stigt24–Reference Sathyaprabha, Kapavarapu, Pall, Thennarasu and Raju26 Both restrictive and obstructive patterns of involvement have been reported, with the improvement of the former with C/L. Reference Monteiro, Souza-Machado, Valderramas and Melo25 Older age and general anesthesia during surgical emergencies may further worsen the suboptimal respiratory function in PD patients. One of the major intra- and postoperative issues in PD is the risk of aspiration pneumonia. The incidence of postoperative aspiration pneumonia is higher in PD compared to patients without PD, Reference Pepper and Goldstein27 which could be attributed to the impaired cough reflex, Reference Ebihara, Saito and Kanda28 defective motor control for cough, Reference Fontana, Pantaleo, Lavorini, Benvenuti and Gangemi29 and dysphagia in the elderly PD population. Reference Potulska, Friedman, Królicki and Spychala30 Although there are no specific guidelines for managing acute respiratory issues, knowledge of the risk of respiratory complications in PD would keep the surgery and anesthesiology teams better prepared. Airway management may be challenging in PD patients, especially those with sialorrhea and dystonic neck posturing. Although uncommon, there are reports of postoperative laryngospasm and respiratory failure in PD. Reference Wang, Saasouh and Botsford31,Reference von Eckardstein, Sixel-Döring, Kazmaier, Trenkwalder, Hoover and Rohde32 Hence, there should be a low threshold to go for chest X-rays, arterial blood gas analysis, and chest physical therapy if PD patients show signs of respiratory abnormalities.
The anesthesiology and surgical teams should be familiar with the potential cardiovascular comorbidities associated with PD. Dysautonomia is common in elderly PD patients, more so in those with advanced PD. The wide fluctuation of blood pressure during anesthesia should be quickly recognized and persistent hypotension in PD can be managed with the α1-adrenoreceptor agonist phenylephrine. Reference Stirt, Frantz, Gunz and Conolly33 Supine hypertension during surgery can be managed with short-acting intravenous vasodilators or with transdermal nitrates in the perioperative setting. Reference Mustafa, Fessel and Barwise34 Finally, careful evaluation and control of blood volume are key in patients with PD and autonomic failure. Knowledge about patients’ history of dysautonomia is important as trivial pharmacological alterations or surgery-related pain may trigger profound fluctuations in the cardiovascular parameters of PD patients. Reference McGrane, Atria and Barwise35 Some common medications taken by PD patients have the potential to prolong the QT interval, which in the context of general anesthesia can make the patients more vulnerable to arrhythmias. For example, antiemetics such as ondansetron, antipsychotics such as pimavanserin and quetiapine, and antidepressants such as citalopram are associated with QT-prolongations. Hence, the treating teams should be vigilant about the dose of these medications, more so in elderly patients with dysautonomia and other cardiac comorbidities.
Intraoperative Anesthetic Considerations
There is no simple anesthetic regimen for patients with PD and the evidence about the safety of anesthetic drugs is based on case reports or case series. When suitable, regional or central neuraxial block offers several benefits. These include patient cooperation while assessing Parkinsonian symptoms, availability of oral dopaminergic agents during intraoperative phase, no requirement for neuromuscular blocking agents and anticholinergic reversal agents, reduced or no use of systemic opioids. On the contrary, the presence of severe rigidity and tremor in parts of the body not under anesthesia may make positioning and monitoring difficult. General anesthesia would eliminate this disadvantage by abolishing the Parkinsonian symptoms, but at the cost of some other disadvantages (postoperative nausea, vomiting, higher risk of aspiration pneumonia). Hence, in addition to the nature of the surgical procedure, severity and laterality of the preoperative Parkinsonian symptoms should be taken into account while deciding about the type of anesthesia. Because of suboptimal respiratory function associated with PD, anesthesia recovery time and extubation time should be optimized. Table 3 summarizes the medications that should be avoided or should be used with caution because of significant adverse effects or interactions.
Table 3. Summary of the medications that should be preferably avoided because of direct adverse effects or interactions with MAO inhibitor
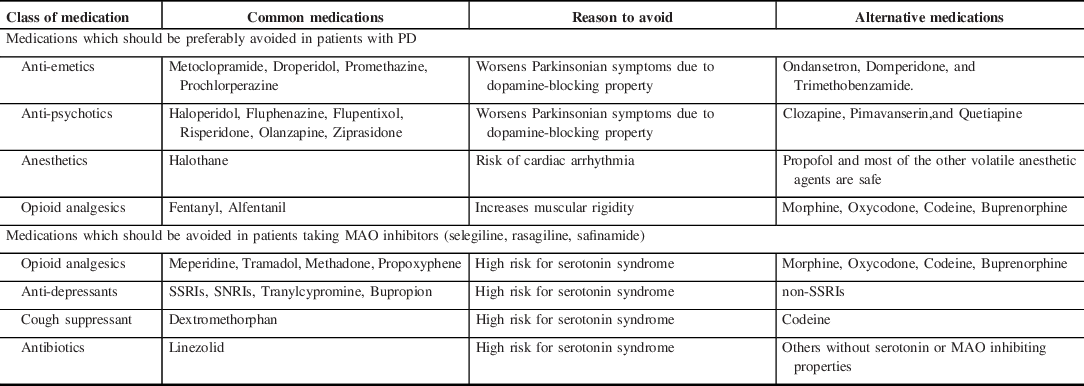
MAO = monoamine oxidase inhibitor; PD = Parkinson’s disease; SNRI = selective norepinephrine uptake inhibitors; SSRI = selective serotonin reuptake inhibitors.
In the case of general anesthesia, a rapid sequence induction is preferred to achieve rapid control of the airway whilst minimizing the risk of regurgitation and aspiration of gastric contents. Propofol is a commonly used anesthetic agent, which is given intravenously both for induction and maintenance. However, there are reports of dyskinesia associated with the use of propofol in patients with and without PD. The emergence of choreic and dystonic dyskinesias was reported after the administration of propofol in two patients undergoing pallidotomy. Reference Krauss, Akeyson, Giam and Jankovic36 Both the patients developed dyskinesia after getting 30 mg bolus of propofol, which subsided within a few minutes after discontinuing propofol in one patient and after giving midazolam to one patient. Although it is unclear how propofol is associated with such involuntary movements, it is speculated that the subtle anti-Parkinson effect of propofol could be contributory. Reference Krauss, Akeyson, Giam and Jankovic36 Although rare, oculogyric crisis Reference Dingwall37 and acute dystonia Reference Zabani and Vaghadia38 were reported with the use of propofol. Despite these adverse effects, propofol remains the anesthetic of choice in patients with PD owing to its rapid onset of action and overall safety. Reference Kalenka and Schwarz39 If it occurs, propofol-induced dyskinesia can subside with the administration of dexmedetomidine. Reference Deogaonkar, Deogaonkar, Lee, Ebrahim and Schubert40 Thiopental should be avoided as it may worsen Parkinsonism and the use of ketamine can be associated with cardiovascular complications. Reference Shaikh and Verma41 The effects of inhalational anesthetic in PD are complex. Although isoflurane may influence dopamine transmission through inhibition of the presynaptic dopamine active transporter (DAT), Reference Votaw, Byas-Smith and Hua42 it is thought to be generally safe in PD. Other inhalational anesthetic agents such as sevoflurane and enflurane can be used in patients with PD, however, intraoperative evaluation and optimization of blood volume are key to prevent hypotension. Halothane should be avoided in PD because of its potential arrhythmogenic effect and the risk of increased cardiac sensitivity to catecholamines. Reference Nicholson, Pereira and Hall43
Opioids can be used as anesthesia adjuncts or primary anesthetic agents for procedures performed under general, regional, or local anesthesia. Opioids should be used with caution in PD because of the risk of respiratory depression, acute dystonic reaction, muscle rigidity, and prolonged postoperative confusion and hallucinations. Reference Nicholson, Pereira and Hall43,Reference Mets44 Patients with PD may be particularly sensitive to opioid-induced muscle rigidity, which has been reported with fentanyl. Reference Mets44,Reference Bailey, Wilbrink, Zwanikken, Pace and Stanley45 Opioid-induced muscle rigidity responds to neuromuscular blockade and naloxone. Reference Buxton, Gauthier, Woo Kinshella and Godwin46,Reference Ahmad and Raza47 The combination of meperidine and selegiline should be avoided as it can provoke agitation, muscle rigidity, and hyperthermia. Reference Zornberg, Bodkin and Cohen48 Non-depolarizing neuromuscular blocking agents are recommended (e.g. rocuronium) as they do not worsen the Parkinsonian symptoms. Reference Shaikh and Verma41 Careful assessment of hydro-electrolyte disorders is important to prevent hyperkalemia with depolarizing neuromuscular blocking agents. Reference Gravlee49
Intraoperative Issues in Patients with Implantable Deep Brain Stimulator
Electromagnetic Interference with the DBS Device
PD patients with DBS devices need additional care during the surgery. This is because of the possibility of electromagnetic interference with the implantable pulse generator (IPG) by the electrical appliances used during surgery and resuscitations, which often include, but not limited to diathermy, electrocautery, external cardiac defibrillator, cardiac monitoring, and electrocardiogram. Electromagnetic interference can damage the IPG, resulting in decreased or increased stimulation or complete cessation of output. There are reports of reversible neurological symptoms and irreversible brain injury which resulted because of the interaction of diathermy and DBS. Reference Roark, Whicher and Abosch50,Reference Nutt, Anderson, Peacock, Hammerstad and Burchiel51 Electrosurgical units usually use two different configurations, i.e. monopolar and bipolar. Given significant adverse effects associated with the monopolar configuration in the context of metallic implants, it is prudent to use only the bipolar electrosurgical device. Reference Weaver, Kim and Torres52 In addition, use of the relatively newer options such as PLASMABLADE and HARMONIC scalpels have good track records of safety and may be considered when available. Reference Epstein, Mayer and Duncan53–Reference Yeoh, Manninen, Kalia and Venkatraghavan55
To summarize, the IPG should be turned off during the surgery, diathermy should be avoided, and bipolar electrosurgical devices should be preferred over the monopolar devices. At the end of the procedure, the IPG should be turned on to the baseline settings. If surgery was performed under general anesthesia, the IPG should ideally be turned on before the reversal of anesthesia to avoid the recurrence of Parkinsonian symptoms when the patient is awake.
Postoperative Considerations
ReInitiation of the Anti-Parkinsonian Medications
Unless contraindicated from the surgery standpoint, the oral anti-Parkinsonian medications should be resumed at the earliest. However, several postoperative issues prompt alterations to the treatment regimen. For example, in PD patients with postoperative delirium, it would be appropriate to withhold medications that may precipitate delirium. These include amantadine and dopamine receptor agonists such as pramipexole and ropinirole. In such scenarios, short-term augmentation in the dose of C/L may be considered up to a dose similar to the preoperative levodopa equivalent dose (LED). In certain gastrointestinal surgeries, neither oral feeding nor nasogastric (NG) or orogastric (OG) tube feeding is possible several hours after the surgery. Such situations warrant judicious use of non-oral alternatives (with rotigotine patch, apomorphine injections) suggested above in the preoperative section.
Several situations may warrant feeding only through NG/OG tube temporarily or through gastrostomy tubes (G-tubes) permanently in patients with advanced PD. Anti-Parkinsonian regimens may be modified in those cases as several formulations (capsules, extended-release preparations) are not recommended through the NG/OG/G-tubes. The nursing team should be instructed to discontinue tube-feeding at least 30 min before and after giving C/L as interaction with protein-rich food reduces its absorption. Similarly, co-administration with vitamin-B6 should be avoided as the vitamin B6 accelerates the systemic metabolism of levodopa. Reference Mars56
Pain Control
Pain related to the recent surgery should be differentiated from the pain sometimes associated with Parkinsonism. Pharmacological therapies such as paracetamol and nonsteroidal anti-inflammatory drugs should be considered first-line for postsurgical pain if there is no contraindication. Opioids should be used with caution in PD, and opioids with SSRI properties should be avoided in those taking monoamine oxidase B (MAO-B) inhibitors (selegiline/rasagiline/safinamide). Such opioids include tramadol, methadone, dextromethorphan, and propoxyphene. Co-administration with the MAO-B inhibitors may cause heightened serotonin activity (serotonin syndrome) which encompasses symptoms such as agitation, rigidity, diaphoresis, hyperpyrexia, and even death. Safer analgesics (without SSRI property) include morphine, codeine, oxycodone, and buprenorphine. Special care needs to be taken for PD patients, as these patients can have worsening of constipation with the opioid analgesics and increased risk of delirium, and pulmonary complications secondary to opioids induce respiratory depression.
Nausea and Vomiting
Nausea and vomiting are common in the postoperative phase. Regarding antiemetic agents, ondansetron, trimethobenzamide (FDA-approved for postoperative nausea), and domperidone (not available in the USA) are preferred in patients with PD. Trimethobenzamide was effective in ameliorating nausea and vomiting in patients on subcutaneous apomorphine (in the first 8 weeks) without any significant adverse effect. Reference Hauser, Isaacson and Clinch57 Given their dopamine receptor blocking properties, medications such as metoclopramide, prochlorperazine, and promethazine should be avoided. Reference Kim, Cheon and Suh58
Postoperative Delirium and Psychosis
The prevalence of postoperative delirium in PD ranges from 11% to 60%. Such variability could be explained by differences in the criteria used for the diagnosis of delirium. Reference Lawson, McDonald and Burn59 The spectrum of manifestation of delirium is wide and previous studies have reported confusion, disorientation, hallucinations, agitation, hypomania as symptoms of delirium. Reference Lawson, McDonald and Burn59 Delirium has an association with length of hospitalization, motor severity of PD, and older age. Reference Lawson, McDonald and Burn59,Reference Lawson, Richardson, Yarnall, Burn and Allan60 A study that investigated the non-motor correlates of postoperative delirium in PD patients undergoing spinal surgeries reported hyposmia and rapid eye movement sleep behavior disorder (RBD) as independent risk factors for delirium. Reference Kim, Kang and Shin61 As delirium can substantially worsen the overall outcome of PD patients, it needs effective prevention strategies (described in the preoperative section) and prompt management. One of the foremost steps in treating delirium is removal of the offending factors, especially the medications that could precipitate delirium. These include certain anti-Parkinsonian medications such as amantadine, dopamine receptor agonists, MAO-B inhibitors, and anticholinergics. Use of opioid analgesics and fluoroquinolone antibiotics should also be carefully monitored. Use of clozapine, pimavanserin, and quetiapine may be considered for patients with florid psychotic symptoms. Reference Ebersbach, Ip and Klebe62,Reference Lenka, Gomathinayagam and Bahroo63
Prevention of Postoperative Infections
Large retrospective studies have reported a significantly higher incidence of postoperative infections in PD patients, compared to those without PD. Reference Huang, Chou and Yeh5,Reference Pepper and Goldstein27 Aspiration pneumonia and UTIs are the commonly reported infections. Impaired swallowing and poor cough reflex in the setting of general anesthesia results in suboptimal airway protection, increasing the risk of aspiration pneumonia. Hence, strict precautions should be taken to prevent aspiration pneumonia, which is a common cause of death among PD patients. Reference Matsumoto, Sengoku, Saito, Kakuta, Murayama and Imafuku64,Reference Pennington, Snell, Lee and Walker65 Early interventions through the speech and language pathologists, chest physical therapy, and incentive spirometry are recommended postoperatively. Bladder dysfunction, often compounded with benign hyperplasia of the prostate in elderly PD patients, may be associated with UTIs in PD patients. Reference Sakakibara, Panicker, Finazzi-Agro, Iacovelli and Bruschini66 If such patients develop urinary retention or incontinence, the threshold for urology consultation should be low. Postoperative urinary retention (POUR) is common with a reported incidence of 5%–70% in the general population. Reference Agrawal, Majhi and Garg67 Although there is no data on the incidence of POUR in PD, the presence of clinical/subclinical dysautonomia would place the older PD patients at a higher risk of having POUR. POUR would require urethral catheterization which, in turn, increases the risk of UTIs. Reference Tambyah and Oon68 Hence, prompt identification and management should be done with the help of the urologists. Any signs of infection in the postoperative phase should prompt urinalysis, and if needed, urine culture to rule out UTI.
Multidisciplinary Approach
Because of the wide variety of both motor symptoms and NMS and the risk for perioperative morbidity in PD patients, a multidisciplinary approach is a key to provide optimal management in the perioperative setting. Importantly, treatment plans should be tailored to the needs of each patient. Timely administration of the right anti-Parkinson medications with the right dose and frequency is not as simple as it sounds. Adequate coordination between the admitting team, nurses, and pharmacy would prevent the medication reconciliation and administration errors. An integrated care approach is viable and should be emphasized, through the contribution of the nursing staff and different specialties such as neurology, anesthesiology, internal medicine, and sometimes cardiology, urology, or gastroenterology. Finally, early involvement of physiotherapy, occupational therapy, and speech-language therapy may help PD patients to avoid deconditioning while hospitalized and reduce the risk of postoperative complications such as aspiration pneumonia. Figure 1 summarizes the common perioperative issues in patients with PD.

Figure 1. Summary of the potential perioperative challenges associated with the care of patients Parkinson’s disease.
Conclusion
Patients with PD are at a high risk of complications in the perioperative setting. A multidisciplinary approach is pivotal to prevent and manage those complications. Accurate medication reconciliation during admissions, continuation of appropriate dopaminergic agents up to the level of pre-hospitalization LED, and prompt use of the alternative routes of drug administration are the keys to an uneventful preoperative phase. Turning off the DBS-IPGs just before surgery, avoiding the diathermy and monopolar surgical appliances, opting for the appropriate anesthetic agents, and prompt identification and management of respiratory and cardiovascular complications are important in the intraoperative period. Eventually, the management in the postoperative phase should focus on adequate management of pain, nausea, and vomiting, prevention of delirium, aspiration pneumonia, and UTI, and early rehabilitation. These approaches are important for safe hospitalization and better clinical outcome of PD patients undergoing surgeries.
Conflicts of Interest
The authors declare that there are no conflicts of interest relevant to this work.
Statement of Authorship
-
(1) Research Project: A. Conception, B. Organization, C. Execution.
-
(2) Manuscript: A. Writing of the First Draft, B. Review and Critique.
-
AL: 1 A, 1B, 1C, 2A.
-
SOM: 1 A, 1B, 1C, 2A.
-
GL: 1B, 1C, 2B.
-
FLP: 1C, 2B.
-
Disclosures
Drs. Lenka, Mittal, and Lamotte have no disclosures to report.
Dr. Pagan reports receiving personal fees and other support from Acadia, AbbVie, Kwowa-Kirin, Accorda, and Adamas; personal fees from Teva; grants from Medtronic; grants, personal fees, and other from the US World Meds; grants from the National Institutes of Health, National Institute on Aging; grants from the Alzheimer’s Association and National Institutes of Health as co-principal investigator, and Parkinson’s Foundation, and personal fees and other from Sunovion and Merz outside the submitted work. Dr. Pagan is a co-founder and has equity in KiefRx.






