Opsoclonus-myoclonus syndrome (OMS; also known as opsoclonus-myoclonus-ataxia or “dancing eyes-dancing feet” syndrome) is a rare, often paraneoplastic neurological disorder, whose hallmark symptoms are opsoclonus, myoclonus, ataxia, and encephalopathy. Reference Wong1 Opsoclonus is a striking oculomotor phenomenon characterized by chaotic ocular saccadic movements that occur synchronously in multiple directions and are involuntary. Reference Wong1 Here, we present a case of OMS that occurred in association with non-small cell lung cancer (NSCLC).
The patient is a 73-year-old woman who initially presented with dizziness and diplopia, which began during the week prior to presentation. She had a past medical history of mild expressive aphasia secondary to a previous left basal ganglia infarct, hypertension, hyperlipidemia, and type 2 diabetes mellitus. She also had a 60-pack-year history of smoking. Neurological exam showed opsoclonus (Video, Figure 1 Panel A ) and a faint, fine postular tremor of the left hand. No myoclonus or ataxia was appreciated at the time. Renal, liver, and thyroid function tests were unremarkable. An MRI of the brain with and without contrast was obtained and revealed bilateral temporal lobe T2 hyperintensity (Figure 1 Panel B), concerning encephalitis. CSF studies revealed 32 nucleated cells/µL (reference value, < = 5), a protein level of 48 mg/dL (reference value, < = 35), and a normal glucose. Infectious studies were negative and included blood cultures, serum HIV screen, and CSF testing for HSV 1/2, VZV, EBV, Enterovirus, Tropheryma whipplei, West Nile virus, and Powassan virus. CSF and serum autoimmune and paraneoplastic antibody panels were negative. FDG-PET of the body demonstrated a large right lung mass (Figure 1 Panel C), with biopsy revealing poorly differentiated NSCLC. The patient was treated with glucocorticoids (1 g of IV methylprednisolone daily for 5 days) and plasmapheresis (5 sessions) for paraneoplastic encephalitis, as well as with concurrent chemotherapy. Despite these treatments, her condition unfortunately worsened, with development of visual agnosia, expressive aphasia, autonomic dysfunction, and bilateral arm myoclonus. She was later discharged home on hospice care and passed away soon after this.
Given the patient’s opsoclonus and later development of myoclonus and encephalopathy in the setting of newly diagnosed NSCLC, with negative infectious studies and other workups, the most likely diagnosis, in this case, is paraneoplastic OMS (P-OMS). The diagnosis is further supported by the rapid progression of the patient’s symptoms, which is characteristic of P-OMS. Reference Bataller2 The patient did have negative autoantibody testing results, but this is a common finding in P-OMS. In one retrospective cohort study of 114 patients with OMS, 45 of whom had P-OMS, autoantibodies were found in only 11% of patients. Reference Armangué, Sabater and Torres-Vega3 Autoantibodies identified in patients with P-OMS and lung cancer in this study included those against the glycine receptor, Ma2, Zic4, and HNK-1. Reference Armangué, Sabater and Torres-Vega3
The pathogenesis of P-OMS is incompletely understood but is thought to have an autoimmune basis, possibly with both humoral and cell-mediated immunologic mechanisms. Reference Oh, Kim and Dieterich4 What drives the opsoclonus itself is also unclear, though the three leading hypotheses implicate pathologic changes to the cerebellum or brainstem. Reference Oh, Kim and Dieterich4 The first hypothesis posits damage of the omnipause neurons of the pons as the cause of opsoclonus; the second (the “brainstem theory”) proposes that changes in the activity of saccadic burst neurons of the brainstem constitute the mechanistic basis; the third (the “cerebellar theory”) states that disinhibition of the fastigial nuclei of the cerebellum is to blame. Reference Oh, Kim and Dieterich4 Among these, the latter two hypotheses appear to have more evidence in their favor than the first one. Reference Oh, Kim and Dieterich4
If OMS is identified, an underlying etiology should be sought, particularly because this can influence treatment and prognosis of the OMS. Reference Bataller2,Reference Klaas, Ahlskog and Pittock5 Malignancy is the most frequently identified cause of OMS, and evaluation for occult malignancy is an important part of the diagnostic workup in all patients with OMS. Reference Wong1 Neuroblastoma is the malignancy most commonly associated with P-OMS in children, whereas in adults, it is small-cell lung cancer. Reference Wong1,Reference Armangué, Sabater and Torres-Vega3 Our case is unusual in that there are only a limited number of other cases in the literature that report P-OMS occurring in association with NSCLC. Reference Bataller2,Reference Musunuru and Kesari6–Reference Karasaki and Tanaka8 We are not aware of any other cases of P-OMS secondary to NSCLC in which plasmapheresis was utilized for management.
Other etiologies of OMS to consider include infections such as HIV, Lyme disease, and West Nile virus; multiple sclerosis; systemic diseases such as celiac disease and sarcoidosis; metabolic derangements such as hyperosmolar coma; and medications such as lithium. Reference Wong1 It is worth noting that in many instances, no etiology of the OMS is ever identified. Reference Wong1,Reference Klaas, Ahlskog and Pittock5
Treatment of P-OMS is directed at the underlying malignancy as well as the autoimmune mechanisms thought to be responsible for the disorder. Chemotherapy for the malignancy can be beneficial not only for its antineoplastic effect but also because of induced immunosuppression. Reference Klaas, Ahlskog and Pittock5 Apart from chemotherapy, immunomodulatory medications that have been used with success in OMS include glucocorticoids, rituximab, and intravenous immunoglobulin (IVIG). Reference Oh, Kim and Dieterich4 Plasmapheresis can be considered for patients who are refractory to IVIG or glucocorticoid monotherapy (as was the case with our patient). Reference Oh, Kim and Dieterich4 Even with appropriate treatment, the prognosis of P-OMS is often poor. Reference Bataller2
Our case illustrates how the isolated neurological finding of opsoclonus can aid in the discovery of a serious underlying condition, and it showcases that OMS can occur even with malignancies, such as NSCLC, which are not commonly associated with OMS. It also illustrates that myoclonus may not manifest until days after the onset of opsoclonus. Finally, our patient’s clinical deterioration over the course of just weeks, considered alongside data showing poor outcomes in a significant proportion of those with P-OMS, suggests that progression of neurological symptoms while on active cancer treatment and immunosuppression should prompt consideration of goals of care discussion or, if appropriate, escalation of therapy. Reference Bataller2
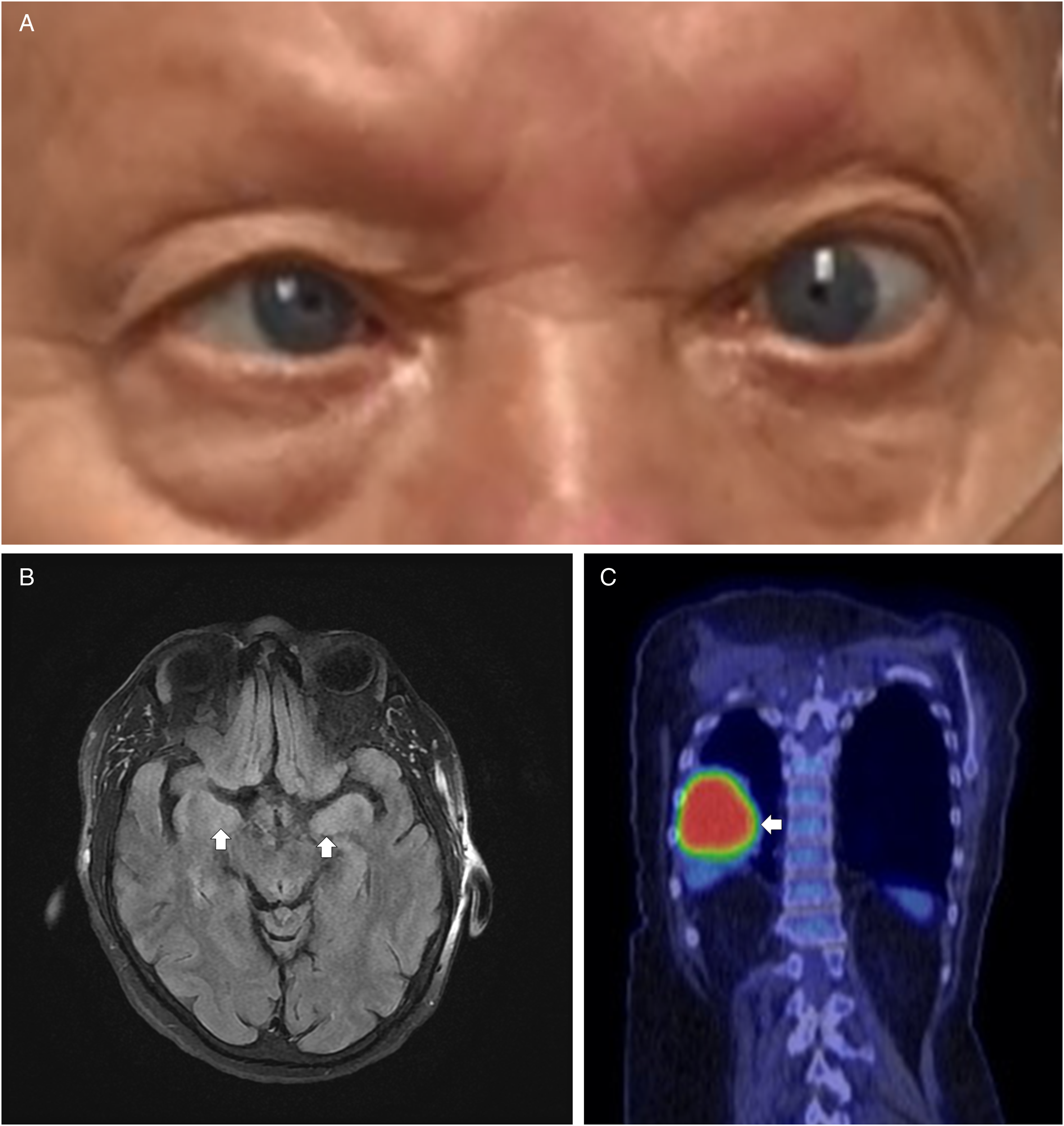
Figure 1: Clinical findings in a 73-year-old woman with paraneoplastic opsoclonus-myoclonus syndrome. a , Still image of opsoclonus footage showing dysconjugate gaze. b , MRI brain T2-weighted sequence showing bilateral temporal lobe hyperintensity (arrows), suggestive of encephalitis. c , FDG-PET imaging demonstrating a large right lung mass (arrow), later diagnosed as poorly differentiated non-small cell lung cancer.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/cjn.2023.329.
Statement of authorship
Dr Bayless reports no disclosures relevant to this manuscript.
Dr Itoh reports no disclosures relevant to this manuscript.
Dr Mustafa has consulted with Horizon Therapeutics regarding educational content for neuromyelitis optica spectrum disorders and neurohospitalist practice. He is an editorial board member of The Neurohospitalist.
Dr Bayless: writing – original draft, writing – review and editing, investigation.
Dr Itoh: conceptualization, writing – review and editing.
Dr Mustafa: writing – original draft, writing – review and editing, supervision.



