Palatal tremor (PT), an uncommon disorder, is a continuous rhythmic, involuntary contraction of the soft palate and is classified into essential PT (EPT) and symptomatic PT (SPT) types. Lesions in the Guillain Mollaret triangle (GMT) are implicated in PT. Clinical studies emphasizing magnetic resonance imaging (MRI) observations, electrophysiological features, and genetic etiologies are infrequent.Reference Deuschl, Mischke, Schenck, Schulte-Mönting and Lücking 1 , Reference Samuel, Torun, Tuite, Sharpe and Lang 2 We describe 27 consecutive patients with PT seen in a single neurology unit over 4 years and review the English literature for available evidence regarding its mechanisms and treatment.
Clinicodemographic data, laboratory investigations, and brain MRI observations were noted. Surface electromyogram (EMG) was recorded wherever possible from the soft palate referenced to the jaw (Neuropack X1 MEB-9200K, Nihon Kohden Corporation, Tokyo, Japan; sweep speed=50 ms/div, sensitivity=100 µV/div, high-pass filter=50 to 100 Hz, low-pass filter=10 to 50 Hz). Brain MRI was acquired using a 1.5 or 3.0 scanner. Guidelines laid down by the Institute Ethics Committee were adhered to. Informed consent was obtained from all patients. The data were incorporated into a Microsoft Excel spreadsheet for analysis.
The clinical features, key laboratory findings, and final diagnosis of our cohort (n=27; male:female=16:11; mean age at evaluation: 37±14.7 years [range, 9-57 years]) are summarized in Table 1 and the Supplementary Table. Patients were classified into SPT (n=26) and EPT (n=1). Patients with EPT reported intermittent clicking tinnitus audible to the external observer and could initiate/suppress the click by adopting specific head positions and pressure over the thyroid cartilage. The click was present for variable duration in sleep. There were no other neurological deficits, and brain MRI was normal.
Table 1 Clinical Features and Brain Magnetic Resonance Imaging Observations in Patients with Palatal Tremor (n=27)
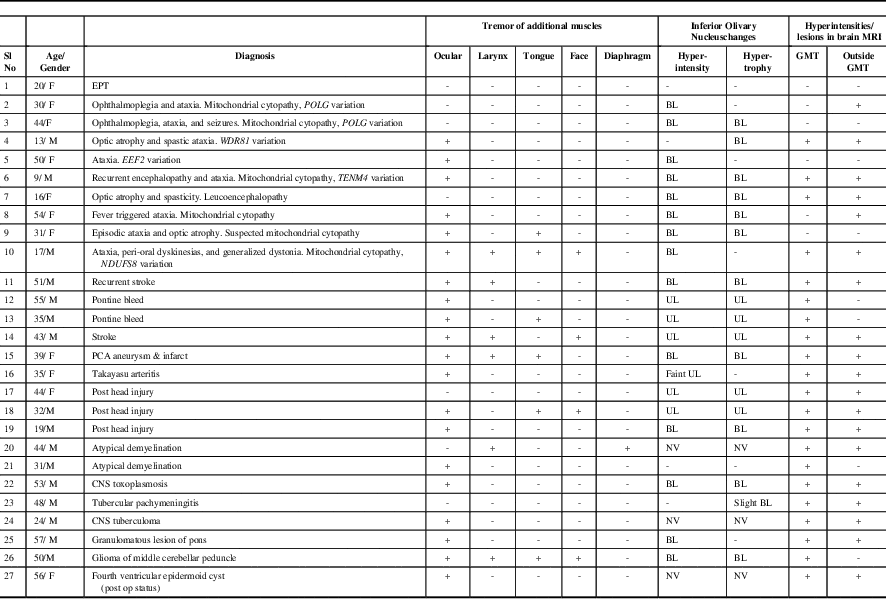
BL: Bilateral; CNS: Central Nervous System; EEF2: Eukaryotic Translation Elongation Factor 2;EPT: Essential Palatal Tremor; GMT: Guillain Mollaret Triangle; MRI: Magnetic Resonance Imaging; NDUFS8: NADH:ubiquinone oxidoreductase core subunit S8; NV: Not Visualized; PCA: Posterior Cerebral Artery; POLG: Polymerase Gamma; TENM4: Teneurin Transmembrane Protein 4; UL: Unilateral; WDR81: WD Repeat-Containing Protein 81
Additional findings in SPT included synchronous contraction of ocular (n=20), laryngeal (n=6), lingual (n=6), facial (n=5), and diaphragmatic (n=1) muscles (Supplementary Videos 1-5). EMG recording of SPT was carried out in ten patients and it showed 2- to 10-Hz slightly arrhythmic bursts. Etiologies of SPT were genetic (n=9), vascular (n=6), trauma (n=3), infections (n=3), atypical demyelination (n=2), posterior fossa tumor (n=2), and granulomatous lesion of the brainstem (sarcoidosis) (n=1). Exome sequencing in patients suspected to have genetic etiology showed variations in POLG (n=2), and in WDR81, TENM4, EEF2, and NDUFS8 genes in one patient each.
Brain MRI showed abnormalities of the inferior olivary nucleus (ION) in the form of: (1) hyperintensity with hypertrophy (bilateral=10, unilateral=5) and (2) hyperintensity without hypertrophy (bilateral=4, unilateral=1). In three patients, ION could not be assessed because of lesions in the brainstem that were large and/or contiguous with ION. Additional observations included signal changes involving (1) brainstem in the region of GMT as well as a region outside the GMT (n=17), (2) brainstem in the GMT only (n=4), (3) a region outside the GMT only (n=2), and (4) normal (n=4) (Figure 1).

Figure 1 (A) Brain MRI in a 20-year-old woman with EPT shows normal findings without hyperintensity or enlargement of ION in axial T2-weighted (T2W) sections. (B&C) Brain MRI and PT recording of a 44-year-old woman with POLG1 variation shows (B) hyperintensity and enlargement of bilateral ION with cerebellar atrophy in axial T2W section of brain and (C) PT recording shows an arrhythmic tremor occurring at a frequency of 2.5 to 5.0Hz. (D&E) Brain MRI of a 35-year-old man with stroke and delayed ataxia shows (D) hyperintensity and enlargement of right ION in axial T2W sections and (E) bleed in the pons extending into the right cerebellar peduncle and hyperintensity in the right ION in coronal T2W sections. (F&G) Brain MRI of a 24-year-old man with CNS tuberculosis shows conglomerate ring enhancing lesions in the left middle cerebellar peduncle and pons with peri-lesional edema, distorting the fourth ventricle in (F) axial T2 and (G) contrast enhanced T1W sections. In this patient the ION could not be visualized. (H-L) Brain MRI and PT recording of a 43-year-old man with stroke shows (H) hyperintensity and enlargement of right ION in axial FLAIR section, while (I) coronal and axial (J, K) T2W sections of brain show infarcts in the left superior cerebellum, right pons, and bilateral thalami, and (L) PT recording shows an arrhythmic tremor occurring at a frequency of about 3.0 to 5.0 Hz. [Surface EMG recording of PT (C&L): Active and reference electrodes were placed over the soft palate and lower jaw respectively. EMG bursts correspond to PT. Sweep speed: 0.2 sec/div, sensitivity: 50µV/div.]
In the present study, the relatively high proportion of patients with genetic causes could be due to referral bias because we are actively involved in research in mitochondrial and other neurogenetic disorders (Supplementary Data). Patients 2 and 3, with ataxia, ophthalmoplegia, and palatal tremor, had variations in POLG1. Although patient 2 had compound heterozygous variations (W478+E1143G), patient 3 had homozygous variation (W478S). The W478 variant in POLG1 is consistently associated with the clinical phenotype of ataxia and PT in the European population, either in the homozygous state or in the compound heterozygous state with the polymorphism E1143G.Reference Nagappa, Bindu and Taly 3 Patient 4 had homozygous variation in WDR81, which has been previously reported in families with autosomal recessive cerebellar ataxia, mental retardation, and disequilibrium syndrome type 2 (Online Mendelian Inheritance in Man [OMIM] #610185). The presence of oculopalatal tremor in our patient expands the clinical spectrum of WDR81 mutations. Patient 10 had juvenile-onset Leigh syndrome and compound heterozygous variation in the gene encoding the NDUFS8 subunit of complex I of the mitochondrial respiratory chain; this was further supported by the presence of isolated complex I deficiency in skeletal muscle. Homozygous and compound heterozygous variations in NDUFS8 are recognized causes of Leigh syndrome (OMIM #256000).
We have earlier reported the association of inferior olivary hypertrophy (IOH) with Leigh syndrome and underlying SURF1 variation, but examination for PT could not be carried out because the cohort consisted of children only. PT associated with mitochondrial disorders and mutations in POLG and SURF1 has been reported. We believe that the presence of IOH in an appropriate clinical setting may provide clues for an underlying mitochondrial etiology.Reference Bindu, Taly and Sonam 4 Patients 5 and 6 had heterozygous variations in EEF2 and TENM4, respectively, that are associated with autosomal dominant spinocerebellar ataxia-26 (OMIM #609306) and hereditary essential tremor (OMIM #616736). Because our patients’ phenotypes did not conform to that described in the literature, the causal role of these variations remains to be established. Other heredodegenerative causes for IOH and PT reported in the literature include spinocerebellar ataxia, Alexander disease, neuroferritinopathy, GM2 gangliosidosis, and progressive ataxia palatal tremor, among others.Reference Nagappa, Bindu and Taly 3 , Reference Borruat 5
Earlier reports indicate that SPT develops after a varying interval of a few weeks to several years following brainstem injury. Anatomical changes evolve sequentially in the ION.Reference Borruat 5 Increased signal in the ION in T2 and proton density sequences is followed by hypertrophy; this coincides with the development of PT.Reference Samuel, Torun, Tuite, Sharpe and Lang 2 Three patients with infarcts in our cohort reported an interval of 1.5 to 10 months between the initial stroke and onset of ataxia. We presume that this may be the interval needed for IOH to develop after brain stem injury and therefore PT. This is a putative statement; we have not examined these patients serially. Pathologically, six stages are described in the evolution of IOH that correlate with MRI changes.Reference Goto and Kaneko 6 The spectrum of MRI changes in ION noted in our study may be a reflection of different stages in evolution of IOH; we have only cross-sectional data on the clinical features and MRI changes.
In our study, nystagmus synchronous with PT, i.e., oculopalatal tremor, was noted in 20 patients. Ocular movements may be vertical and pendular or horizontal and torsional and may be symmetric or dissociated.Reference Kim, Moon, Choi, Kim and Sharpe 7 Oculopalatal tremor produces oscillopsia, which is sometimes distressing.Reference Borruat 5 PT may also be associated with rhythmic movements of face, larynx, and diaphragm, as was noted in a few patients in our study. Rarely, vocal cord and facial tremor have been described.Reference Samuel, Torun, Tuite, Sharpe and Lang 2 Deuschl et al. reported that the frequency of PT was 120 to 130 jerks per minute, with variable involvement of the pharynx, diaphragm, vocal cords, face, and eyes.Reference Deuschl, Mischke, Schenck, Schulte-Mönting and Lücking 1 Electrophysiological recordings in subsequent studies showed a much lower frequency of 2 Hz.Reference Samuel, Torun, Tuite, Sharpe and Lang 2 In our study, PT was arrhythmic and the frequency ranged from 2 to 10 Hz.
The GMT is formed by fibers that link the dentate nucleus with the contralateral red nucleus, central tegmental tract, and ION.Reference Borruat 5 Increased glucose metabolism in the medulla, encompassing the region of ION and increased blood flow to the ION and dentate nucleus, have been demonstrated.Reference Borruat 5 The “dual mechanism” hypothesis elucidates the role of ION and deep cerebellar nuclei in PT. ION is the “pacemaker” oscillator. A lesion in the GMT causes transsynaptic deafferentation, loss of inhibitory λ-aminobutyric acid afferents, and compensatory increase in presynaptic terminals of deafferented ION.Reference Borruat 5 Electrotonic coupling of dendrodendritic gap junctions facilitates synchronized oscillations of neurons in ION. Further, abnormal soma-somatic gap junctions in IOH increase the strength of electrotonic coupling. Specific T-type calcium channels that are expressed on neurons of ION modulate their oscillations. The low amplitude signal from the ION is subsequently amplified by the deep cerebellar nuclei.Reference Borruat 5
IOH may occur without a lesion in the GMT and may or may not be associated with a lesion outside the GMT.Reference Gu, Carr, Kaufmann, Kotsenas, Hunt and Wood 8 In the present study, abnormal ION in the form of hyperintensity with or without enlargement was the isolated observation in three patients, whereas, in another two subjects, a lesion was noted, but outside the GMT. Thus neuronal pathways outside the GMT may have a role in IOH. Earlier instances of PT developing in the presence of cortical lesions without brainstem or cerebellar involvement highlighted the role of cerebral cortex in the generation of IOH and PT.Reference Tatum, Sperling and Jacobstein 9 In our cohort, two patients did not have ION abnormality; thus, PT may develop without MRI changes.Reference Samuel, Torun, Tuite, Sharpe and Lang 2 Besides this, PT as a clinical correlate is seen in only a proportion of subjects with IOH.Reference Carr, Hunt, Kaufmann, Kotsenas, Krecke and Wood 10 We did not assess other patients with IOH who did not have PT. To add to the enigma is the observation made in other studies that IOH that develops after stroke or trauma and eventually resolves, whereas PT persists.Reference Samuel, Torun, Tuite, Sharpe and Lang 2 In vivo studies delineating connectivity of ION to GMT as well as other areas of brain and spinal cord in health and disease is now possible using advanced MRI techniques such as diffusion tensor imaging and functional MRI.Reference Borruat 5 This may shed light on this intriguing topic.
In conclusion, we report a fairly large cohort of patients with PT from a single center. PT is a movement disorder known for its nosological diversity and etiological heterogeneity, linked by a common anatomical substrate (i.e. the ION). We highlight newer etiologies including uncommon genetic variations underlying PT. A spectrum of changes was noted in ION ranging from unilateral or bilateral hyperintensity with or without hypertrophy to normal findings.
Acknowledgments and Funding
The study is supported in part by a grant from Indian council of Medical Research to PSB (Grant No. 54/9/2012-HUM-BMS).
Disclosures
PSB discloses support from the Indian council of Medical Research (grant no. 54/9/2012-HUM-BMS). The remaining authors have nothing to disclose.
Supplementary material
To view material/s referred to in this article, please visit https://doi.org/10.1017/cjn.2017.273




