Introduction
In Canada, 19 people die every day due to opioid overdose. In addition, there are 16 hospitalisations every day due to opioid poisoning.1 Despite increased efforts of government and non-government organisations to establish harm reduction programmes and education initiatives, the opioid crisis continues to worsen. The crisis has been attributed to a number of factors, including an increasingly toxic drug supply, limited social services for people who use drugs, unaffordability of housing and lack of support for mental health. These factors lead to stress, isolation, and anxiety, all of which have been exacerbated by the COVID-19 pandemic.1–Reference McGuire, Aulisio and Davis5 In the province of British Columbia (BC) alone, overdose deaths in 2021 were the highest ever recorded.6 These trends parallel global statistics. In the United States for example, a record 100,000 people died due to opioid overdose in 1 year of the COVID-19 pandemic.Reference Dyer7 Australia and New Zealand are similarly struggling with the opioid crisis, where the drug mortality rate (100 per million population) is more than 2.5 times the global average.8 The economic burden of OUD on the health care system, in lost productivity, and law enforcement is $78.5 billion and $3.5 billion per year in the United States and Canada, respectively.Reference Sanyal9 Overall, the World Health Organization estimates that 585,000 people die each year due to drug use, with 70% of those deaths being caused by opioids.10
Opportunities for Action
• Engage with key stakeholders – opioid users, clinicians, scientists, ethicists and policy- and law makers – to explore and inform the future of neuromodulation for OUD.
• Collaborate with Indigenous communities to identify priorities on the landscape of neuromodulation for OUD and integrate Indigenous voices and knowledges to advance developments in a culturally appropriate way.
• Promote the proactive consideration of practical neuroethics principles to inform the design and development of large clinical trials investigating the effectiveness of neuromodulation for OUD in Canada.
• Provide neuroethics leadership in global neuroscience for the evolution of neuromodulation for OUD that is equitable, accessible and based on evidence-informed policy.
Neurotechnologies such as deep brain stimulation (DBS) and repetitive transcranial magnetic stimulation (rTMS) have gained traction as treatments for addiction in various countries such as Germany, the Netherlands, the United States and China.Reference Luigjes, van den Brink and Schuurman11 However, these treatment options have been met with apprehension from both clinicians and patients, presumably owing to fear, stigma, reluctance to label addiction as a brain disorder and different perceptions of the acceptability of invasive (surgical) and less or noninvasive (not surgically penetrating the scalp) interventions with varying degrees of reversibility.Reference Bari, DeCisare and Babayan12 Further complicating this landscape are socio-demographic factors, as marginalised communities are disproportionately affected by addiction have poor access to care and distrust the health system due to past experiences of racism and other forms of structural violence.Reference Baah, Teitelman and Riegel13 This disproportionate representation is reflected, for instance, in a report by the First Nations Health Authority of BC that shows that Indigenous people in the province are dying due to illicit drugs at a rate 5.3 times higher than non-Indigenous people.14 The crisis within Indigenous populations is worsening as well, with a 119% increase in toxic drug deaths in 2020 when compared to the previous year.14
To date, the most common treatments for OUD involve medication-assisted therapies (MATs) such as methadone maintenance treatment (MMT), ideally administered alongside any of the various forms of psychotherapy and behavioural counselling. However, counselling services are expensive, resources are overburdened and funding for subsidised services for OUD fluctuate, leaving many of those seeking treatment to rely solely on pharmacological approaches.Reference Nadeau15–Reference Palepu, Gadermann and Hubley18 MATs present their own limitations and complications, with most critiques emphasising that its intent is not to eliminate the craving for opioids, but rather to replace illicit drugs with a more controlled opioid (e.g. methadone) to control withdrawal symptoms.Reference Fischer, Rehm and Kim17 Once patients begin MAT, many never become fully abstinent of all opioids, but rather relapse or continue on MMT indefinitely. Harm reduction approaches such as MMT are important to eliminate the plethora of risks associated with injection drug use such as various forms of hepatitis, HIV infection and toxicity; however, its success is rooted in population and public health values that emphasise the economic burden of addiction rather than individual and subjective quality of life and cessation of craving.Reference Fischer, Pang and Tyndall16,Reference Fischer, Rehm and Kim17
With a worsening opioid crisis worldwide, there is an imperative to explore all promising treatments for opioid use disorder (OUD), and with the disproportionate burden among marginalised communities, understanding the ethics of implementation is a critical task to ensure that accessibility and equity, alongside cultural meaningfulness, are at the forefront of discussions.Reference Harding, Manora and Marra19–Reference Harding and Illes21 Here, we examine the current state of the science and ethical considerations of neuromodulation for OUD as a case study. We focus on DBS, rTMS, transcranial direct current stimulation (tDCS) and percutaneous electrical stimulation (BRIDGE) because of evidence of their early applications in Europe and Asia, as well as their growing availability in Canada. Electroconvulsive therapy trials have only been reported for alcohol, tobacco/nicotine, or methamphetamine dependence to date; none for OUD. Focus ultrasound has not yet been used in the context of addiction medicine. We conclude by highlighting gaps in the current literature, new ethical considerations and identify future directions for progress in this field.
Case Study
Neuromodulation for OUD
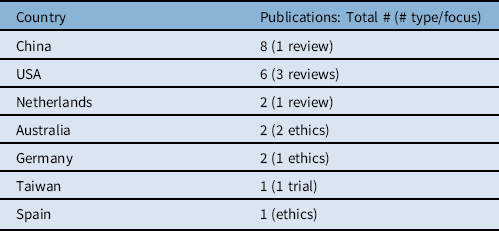
The literature on the effects of DBS, rTMS, tDCS and BRIDGE on dependence such as nicotine, alcohol and stimulants, as well as in the treatment of mental health disorders such as refractory major depressive disorder points to their potential to treat OUD. The literature (Table 1) originates from different countries (Table 2). Nearly 40% (8/22) are from China; 27% (6/22) from the United States. Some describe single or low N case reports; others report on results from larger cohorts. Most are scientific reports of case studies or clinical trials; others are reviews or commentary.
Table 1: Published articles addressing the potential of neuromodulation to treat opioid use disorder. Articles were retrieved from PubMed using the search terms “neuromodulation”, “addiction”, “opioid use disorder”, “heroin addiction”, “rTMS”, “deep brain stimulation”, “tDCS”, “neurotechnology”, “non-invasive”. Returns were manually curated for relevance and duplicates removed

Table 2: Distribution of publications relating to neuromodulation for OUD by country
Invasive interventions: DBS, the sine qua non invasive intervention that does not involve surgical excision of brain tissue, has shown tremendous effectiveness in managing symptoms of Parkinson’s diseaseReference Hardesty and Sackeim22 and treating psychiatric disorders such as depression, anxiety and obsessive compulsive disorder.Reference Kuhn, Lenartz and Huff23,Reference Sturm, Lenartz and Koulousakis24 In the process of researching the effects of DBS on these disorders, Kuhn and colleagues noted that when patients with anxiety disorder underwent DBS of the nucleus accumbens (NAc) area, cravings for and consumption of alcohol were significantly reduced.Reference Kuhn, Lenartz and Huff23 The NAc area is thought to play a significant role in the binge and intoxication phase of addictionReference Bari, DeCisare and Babayan12,Reference Luigjes, Van Den Brink and Feenstra25 and is the basis for subsequent clinical DBS research specifically targeting addiction through stimulation of that region of the brain.
In China in 2011, Zhou and colleagues reported results on the first-known case report of DBS for OUD.Reference Zhou, Xu and Jiang26 The patient, a 24-year-old man, had been intravenous heroin dependent for 5 years and had failed to respond to multiple forms of treatment including detoxification, psychotherapy and behavioral counselling, and MAT. The patient voluntarily underwent DBS implantation of the NAc with stimulation gradually increased from .8V to 2.5V at a frequency of 145 Hz for a period of 2.5 years. At 2.5 years and no drugs since pre-surgery, the stimulation was switched off. At 3 years post-surgery, the implanted device was removed and the patient remained sober for another 3.5 years afterward – a total of 6.5 years sober. There were no reported side effects or personality changes, and the patient experienced significant improvement in memory, depression symptoms, anxiety and IQ. The patient also reported a significant reduction in cigarette consumption and had returned to full-time work.
This early study suggested that DBS could not only be a viable method of managing cravings but also a permanent solution to substance dependence with significant potential to improve multiple measures of quality of life. A similar trial was performed by Valencia-Alfonso and colleagues in the Netherlands with a 47-year-old man who had been dependent on heroin for 22 years.Reference Valencia-Alfonso, Luigjes and Smolders27 Like the patient in the Zhou et al. trial, common treatment methods such as detoxification and MATs had been unsuccessful. Three contact points of bilateral DBS at the NAc were tested: ventral, middle and dorsal. The investigators found that stimulation at the middle points had the opposite of the desired effect, increasing both heroin craving and heroin use. While there were no significant effects when stimulating the ventral points, dorsal stimulation points significantly decreased both heroin craving and use. A stimulation of 3.5V at a frequency of 180 Hz enabled the patient to reduce drug use and ultimately cease heroin use entirely. Contrary to the previous case, this patient did experience one relapse between implantation and the 6-month follow-up; however, the patient regained sobriety by the 6-month follow-up. Of note is that this case report also included experimental recordings of intracranial electroencephalogram (iEEG) to gauge NAc activity when the patient was presented with drug-related pictures. There were significant power differences at the dorsal points between drug-related and drug-unrelated pictures pre-stimulation. On the basis of these findings, the authors propose that iEEG-informed stimulation points can optimise DBS for reducing drug craving and use.
The exploration of DBS to treat OUD continued with another study by Kuhn and colleagues in Germany, where two patients experiencing OUD underwent DBS at the NAc. Both patients were also using other substances, a common occurrence with opioid users.Reference Kuhn, Möller and Treppmann28 Patient 1 was a regular user of alcohol and amphetamines, while Patient 2 used amphetamines and benzodiazepines. A seizure occurred 2 days after surgery in Patient 2 who also had a history of epilepsy. No other adverse effects were noted for either patient.
Unlike previous trials, MAT was used in conjunction with DBS stimulation. Levomethadone was administered to the patients and gradually decreased based on craving scores mapped on a 10-point visual analog scale (VAS). The dose of levamethadone was decreased if the patient scored less than 5. Eventually, both patients were completely tapered off and able to maintain heroin sobriety with Patient 1 remaining abstinent through a 12-month follow-up period and Patient 2 through a 24-month follow-up period. Their use of other drugs (amphetamines, benzodiazepines, alcohol), however, did not diminish and even occasionally increased.
These results contrast with the multiple failed attempts (relapse and continuation of chronic heroin use) to use MATs prior to DBS implantation and confirms previous study findings that DBS at the NAc has the potential to reduce cravings for opioids and lead to cessation of OUD. However, the potential limitations of DBS, especially for patients with comorbid drug use cannot be ignored. Other studies highlight this and other considerations as well.
The first reported death due to overdose after DBS implantation occurred in 2018 when a patient of Zhang and colleagues in China died 3 months post-surgery.Reference Zhang, Huang and Zheng29 The patient had been heroin dependent for 17 years and engaging in MMT for the most recent 7 of them; however, he would relapse with heroin monthly. Implantation was performed at the ventral capsule/ventral striatum area. After initiation of stimulation, the patient reported a decrease in both heroin cravings and heroin withdrawal symptoms, suggesting efficacy of DBS. The patient also significantly reduced his cigarette use, returned to work and reported better sleep. Eventually, all heroin withdrawal symptoms subsided. Between the second and third months following surgery, the patient relapsed with heroin eight times and reported an increase in cravings and withdrawal symptoms. The patient requested higher voltages to manage the increased cravings. Upon increasing the voltages, the patient exhibited symptoms of hypomania, so voltages were decreased. Prior to his death, the patient reported increasing his heroin use to increase the pleasure response he experienced. He also reported feelings of invincibility due to the reduced withdrawal symptoms and cravings that came initially with DBS stimulation.
The authors speculate that this patient’s struggle with anti-social personality disorder may have contributed to the negative outcome of the intervention.Reference Hser, Evans and Grella30 The results overall emphasise the need to consider co-morbid mental health, especially serious psychiatric disorders, in the design of DBS trials and treatment protocols.
To date, Chen and colleagues have performed the largest clinical trial of DBS for OUD.Reference Chen, Li and Ge31 Their study based in China included eight participants, each with at least 3 years of heroin dependence who had not been successful with alternative treatments such as MAT. Patients meeting inclusion criteria for DBS implantation were required to complete a detox programme, with urine analyses and a naloxone challenge test to confirm completion of detoxification. Patients with severe psychiatric disorders or cognitive impairments were excluded from the trial.
Chen and colleagues placed bilateral electrodes at the NAc and anterior limb of the internal capsule (ALIC). Optimization of DBS parameters was performed for a 1-week period following discharge, with voltages and frequencies changed based upon adverse effects, reported heroin cravings, and mood changes. Parameters were assessed every 24 hours. Stimulation was planned for the approximately 2 years of the battery life of the device.
Five patients reached their final follow-up appointments and maintained heroin sobriety (approximately 40 months or 3.5 years). Patients who did not relapse reported a significant reduction in cravings, significantly higher quality of life, and scored significantly lower on psychometric assessments. Patients who relapsed reported similar reductions in cravings and psychometric scores at the times they remained abstinent from heroin use; however, their scores returned to baseline after relapse. Two patients relapsed at months 7 and 10. One patient lost contact with the investigators after 3 months.
The results from this clinical trial showed that DBS at the NAc and ALIC areas may help certain patients remain abstinent from heroin use after completing a detoxification programme prior to DBS implantation. It is important to note that the absence of psychological or pharmacological treatment support after DBS stimulation was initiated for these patients may have contributed to the variability of their clinical trajectories and the trial outcomes.Reference Nadeau15,Reference Brown32
The most recent trial of DBS for OUD and the only one in North America to date was conducted by Mahoney and colleagues in the United States.Reference Mahoney, Haut and Hodder33 The patient was a man in his 30s who had struggled with severe OUD for 10 years. Similar to participants in previous studies, the man had tried several available addiction interventions such as MAT without success. The patient also used benzodiazepines and had experienced four overdoses in the previous year. Mahoney and colleagues implanted bilateral electrodes at the NAc and ventral capsule. Once discharged, the patient continued to be monitored for 12 months in an outpatient setting where DBS parameters could be adjusted for optimisation and clinical assessments related to OUD such as urine toxicology, cue reactivity and cognitive functioning could be administered.
DBS for OUD was found to be a safe procedure in this trial with no adverse events reported. The patient reported complete abstinence from drug use throughout the entire 12-month follow-up period, which was confirmed through urine toxicology. Significant reductions in cravings throughout cue exposure tests were shown post-surgery and continued to reduce throughout the 12-month follow-up period. Positron emission topography results showing an increase in frontal lobe metabolism indicating improved executive functioning corresponded with the patient’s improved performance on a decision-making task test and improved decision-making in life – sobriety and securing employment. In addition, the patient continued to engage positively in alternative therapies for addiction including MAT, attending individual therapy, as well as group support settings.
Non-invasive interventions: Neuromodulation modalities such as rTMS, tDCS and BRIDGE, do not involve surgical intervention and may be considered by different stakeholders to be variously non-invasive,Reference Coates McCall, Minielly and Bethune34 are being tested for disorders such as major depression and gaining traction in addiction medicine today.Reference Spagnolo and Goldman35 The significant advantage of these approaches is that clinical trials can be much larger in comparison to their invasive counterparts.
Shen and colleagues performed a randomised, controlled crossover study with 20 patients to examine the potential of rTMS to treat OUD in China.Reference Shen, Cao and Tan36 Participants assigned to the treatment group (n = 10) underwent 10 minutes of rTMS for a total of 2000 pulses at 10 Hz applied over the dorsolateral prefrontal cortex (DLPFC). A matched control group (n = 10) underwent a similar time protocol but with sham rTMS. After the first session of rTMS, participants in the treatment group showed a significant reduction in heroin craving based on cue-induced craving, while participants in the control group did not. Continuing this treatment protocol for 4 days further reduced craving scores in the treatment group with no changes in the control group.
In a larger clinical trial in China (n = 118) with a longer follow-up period, Liu and colleagues reported a significant reduction in heroin cravings with both low (1 Hz) and high (10 Hz) frequency rTMS over the DLPFC when compared to controls.Reference Liu, Zhao and Liu37 The reductions in cravings were shown to last up to 60 days after the most recent rTMS session. Adverse events included neck pain, headache, and dizziness.
The efficacy of rTMS has also been investigated in combination with other therapies commonly used to treat symptoms of OUD.Reference Krupitsky, Burakov and Dunaevsky38–Reference Pradhan, Parikh and Makani40 For example, Pradhan and Rossi performed rTMS on three patients alongside one infusion of ketamine at 0.75 mg/kg over the course of 45 minutes.Reference Pradhan and Rossi41 Following a one-week washout period, participants then began rTMS and mindfulness sessions. Similar to other studies, the rTMS was performed over the DLPFC at a frequency of 10 Hz for a total of 3000 pulses. Mindfulness sessions involved the Trauma Interventions using Mindfulness Based Extinction and Reconsolidation of memories (TIMBER) protocol. TIMBER uses principles of yoga and meditation in conjunction with those of cognitive behavioural therapy (CBT) to confront harmful memories associated with past trauma.Reference Pradhan, Parikh and Makani40,Reference Pradhan and Rossi41 Five total sessions of rTMS and TIMBER were performed over a two-week period. Following the five sessions, patients showed a 65.7% reduction in craving score, as well as a 41.2% increase in mindfulness score. By contrast, Tsai and colleagues found that rTMS over the DLPFC in addition to MMT did not improve heroin use behaviours or cravings in a cohort of 20 participants in Taiwan, although there was evidence that it did reduce depressive symptoms.Reference Tsai, Wang and Liu42
Using tDCS, Wang and colleaguesReference Wang, Shen and Cao43 attempted to reduce opioid cravings based on prior evidence of its previous effectiveness in reducing nicotine cravings.Reference Fecteau, Agosta and Hone-Blanchet44–Reference Falcone, Bernardo and Ashare46 Twenty males with OUD who were abstinent for at least 1.5 years were randomly assigned to tDCS treatment group or sham tDCS. Electrodes were placed over the left frontal-parietal-temporal area for cathodal stimulation and over the occipital area for anodal stimulation (1.5 mA). The treatment group showed a significant decrease in opioid craving score compared to the control group.
In a final example of noninvasive neurotechnological approaches to OUD, Miranda and TacaReference Miranda and Taca47 investigated the potential of percutaneous electric nerve field stimulation (BRIDGE device) as an alternative to pharmacotherapy to transition to long-term MAT as, they note, using MAT too early without an induction phase can thrust patients into immediate and severe withdrawal.Reference Oviedo-Joekes, Palis and Guh48 The BRIDGE device is an auricular field stimulator that stimulates the peripheral cranial neurovascular bundles in the ear.Reference Frangos, Ellrich and Komisaruk49,Reference Kraus, Kiess and Hösl50 It delivers 3.2V at multiple frequencies, but only has a battery life of five days. In this study, BRIDGE was used with 73 patients who presented to outpatient addiction treatment clinics. All patients had a significant reduction in their Clinical Opioid Withdrawal Score in the 60 minutes after the onset of stimulation. After a five-day period, 64 of 73 patients (88%) were successfully transitioned to MAT. Although this study did not use neuromodulation to directly treat OUD by reducing cravings, it provided evidence that neuromodulation can be used to rapidly decrease the effects of opioid withdrawal and successfully transfer patients to long-term MAT to continue rehabilitation.
Overall, the studies suggest a promise for neuromodulation to treat or aid in the treatment of OUD. They are limited by small sample sizes and strict exclusion criteria, however, and generally do not engage with the ethical implications of the research. We address opportunities to fill this latter gap next.
Opportunities
Alongside the continued drive for neurotechnological solutions to pressing neurologic and psychiatric conditions resides the drive for ethical attention to them. We view this as a critical opportunity on the trajectory of effective, safe and meaningful bench to bedside translation. Indeed, ethical considerations of procedures involving alteration of and implantation in the brain of people with OUD may easily match major physical risks associated with surgery or exceed concerns over minor side effects associated with less invasive procedures (Table 3).
Table 3: Examples of ethical, social, cultural and legal considerations and concerns about neuromodulation for OUD and possible remedies
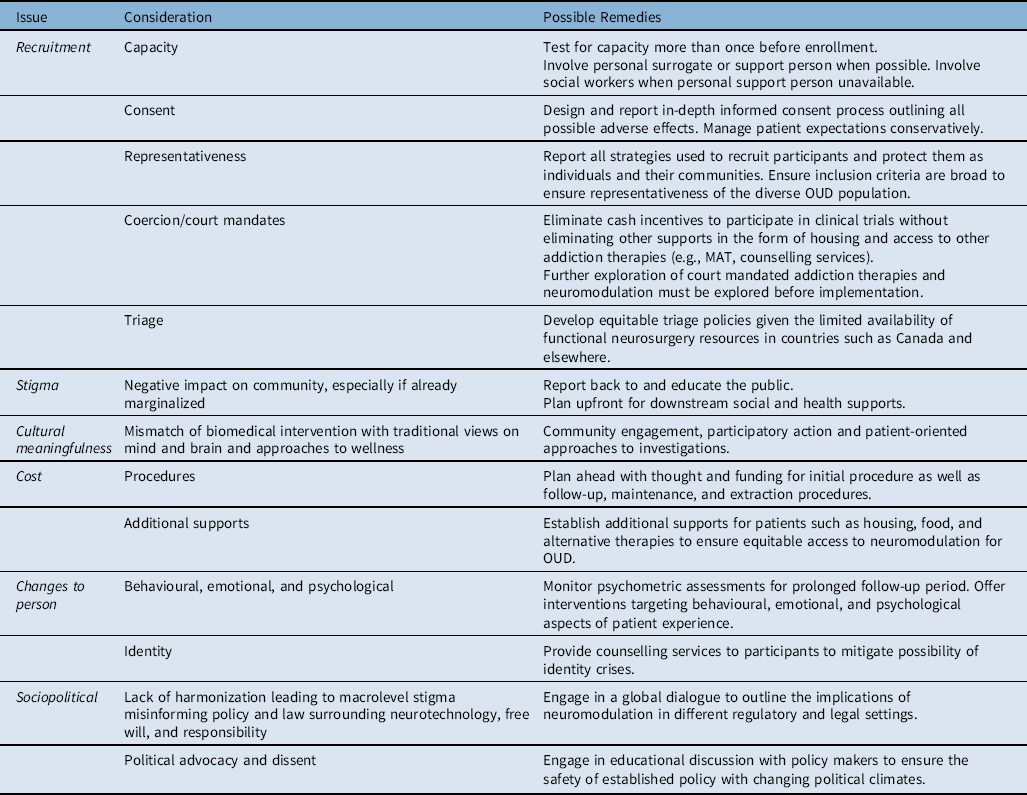
Ethical considerations involve the wide-ranging values and views people possess about brain and mind, and computer interfacing devices that affect agency, responsibility, and the sense of embodiment, estrangement and even identity and personhood.Reference Gilbert, Cook and O’Brien51–Reference Sample, Aunos and Blain-Moraes54 Further complicating the ethics of neuromodulation is the dark history of psychosurgeries of the past, carrying stigma and distrust despite the oversight offered today by institutional review bodies and safety and accuracy advanced by stereotactic guidance.Reference Clausen55 In the context of OUD specifically, coercion to participate and recruitment and retention of an often highly marginalized population that are struggling psychologically, socially and economically are of particular concern. Ensuring equitable access to neuromodulation for OUD regardless of housing status, employment status, available funds, and support circle status must be prioritized for this heterogeneous population. In addition to access, the inclusion and careful design of post-trial multidisciplinary care and longitudinal implant and health monitoring for patients from diverse and marginalized social backgrounds must be thoughtfully and responsibly integrated, even beyond the investigational period in this population. The ethics must evolve, therefore, be carefully aligned with the engineering and development of trials themselves.
ClausenReference Clausen55 argues that, given the uncertainty surrounding the mechanisms of DBS and efficacious target sites, DBS for psychiatric disorders should only be used as an absolute final option, after all alternative therapies involving pharmacological, psychological, and behavioural interventions have been exhausted. Carter and HallReference Carter and Hall56 have been critical of proposals to use DBS to treat addiction, citing the incidence of adverse events after surgery at 11% including serious infections as well as cognitive, behavioural, and emotional side effects.Reference Kleiner-Fisman, Herzog and Fisman57 The authors argue instead for increased access to proven treatments such as pharmacological and psychotherapeutic interventions. They further argue that to continue investigating DBS through clinical trials for OUD, there is an expectation that there is strong pre-clinical evidence of efficacy, evidence of the long-term effects of DBS in psychiatric patients, a strong theory for which brain areas to target, and patients which only have the most severe, debilitating form of addiction. Previous studies on DBS and OUD fail to fulfill these criteria, including only short follow-up periods, uncertainty surrounding target areas, and the inclusion only of patients who had completed detoxification or had begun MAT.
Despite explicitly opposing the use of DBS as a treatment for addiction, Carter and colleagues outline important considerations for future clinical trials. First, patient recruitment is of particular importance, including informed consentReference Carter, Bell and Racine58: patients must have the capacity to understand the risks of the intervention and goals of experiment or trial, have freedom of choice, and have access to all other forms of therapy in clinical equipoise. It is vital that the strategies to achieve these targets are made explicit in all research.Reference Anderson, Eijkholt and Illes59 Patients with refractory addiction who have exhausted all treatment options should be the only patients recruited. In addition, patients who are dependent on substances without effective pharmacological treatments (e.g., cocaine) should be prioritized, as opposed to those with OUD who have access to proven therapies in MMT. The authors do not address that DBS and MAT for OUD target different aspects of this multifaceted disorder, however. While MAT may assist in the withdrawal process and can be tapered in the future, DBS will directly address cravings. Thus, further ethics guidance surrounding the prioritization of differing OUD treatment options must be explored.
In a survey of medical professionals about DBS and treat addiction, Ali and colleaguesReference Ali, Difrancesco and Ho60 found a unique concern about financial incentives for patients in clinical trials of DBS for addiction. Past work has shown that some patients struggling with addiction can see clinical trials as an opportunity for income. Ali and colleagues suggested researchers should reduce or eliminate compensation for participation,Reference Ali, Difrancesco and Ho60 however this may also impact patients who may not have the means or time to participate in this study without compensation. Sustained support, including for the cost of maintenance of any intervention, must be a factor.
Conclusion
Neuromodulatory techniques such as DBS, rTMS, and tDCS show promise in treating OUD, a significant public health crisis in Canada. However, no trials have yet been reported for this country, and reports from others are limited. Moreover, ethical commentary has focused only on DBS to date. There is ample reason to consider that rTMS, tDCS and other evolving neurotechnologies that involve scalp-based or wearable interventions will have overlapping as well as unique implications.Reference Coates McCall, Minielly and Bethune34,Reference Lavazza61–Reference Ford and Henderson65
Although preliminary and early studies contain small sample sizes or individual case reports, results indicate neuromodulation may significantly reduce opioid cravings – an effect absent in traditional approaches such as MAT. The expansion of this research into large clinical trials must be more representative of the general OUD population, implement carefully designed informed consent processes, and make traditional therapies available to participants in conjunction with neuromodulation. The prioritization and explicit integration of ethical, as well as cultural and legal considerations throughout the research process, and global engagement of all affected stakeholders – patients, clinician-innovators, scientists, ethicists, law- and policy-makers – in dialogue will be critical in realizing the potential of this approach in the future. Further ethical guidance will follow the evidence that is to be collected over the coming years.
Author Contributions
The authors jointly conceived and developed the idea. QB conducted the literature search and prepared the manuscript. Both authors contributed to and reviewed the final manuscript.
Author disclosures
QB is supported by a 4 year UBC Doctoral Fellowship Award. No other disclosures. JI received support for this work from the UBC Distinguished Scholars programme and the North Family Foundation scholarship for distinguished professorship in neuroethics. JI also receives support in the form of grants from NIH, CIHR, NSERC, TOSI, ERAnet Neuron, and royalties from Elsevier, Springer and Oxford. No competing interests to disclose.





