Background
During our systematic search for natural product-based anti-protein misfolding agents for the putative treatment of Alzheimer’s disease (AD), we investigated maple syrup. Canada is the world’s largest producer of maple syrup, accounting for 80% of global output; 2013 annual production was 46 million litres. 1 Maple syrup is produced by thermal evaporation of the sap from maple (Acer) trees, a process which concentrates the contained sugars as well as producing a variety of chemical reactions responsible for the distinct colour and taste of the syrup. In addition to the sugars (predominantly α-D-glucopyranosyl-(1→2)-β-D-fructofuranoside), the collected sap contains a wide range of naturally occurring oligosaccharides, amino acids, polyphenols and phytohormones. The prolonged high temperature thermal process involved in producing syrup from sap also results in the formation of a variety of other phenolic by-product compounds. Maple syrup extracts have been reported to exhibit a variety of health-related properties including antioxidant, antimutagenic and anti-cytoproliferative activities.Reference Legault, Girard-Lalancette, Grenon, Dussault and Pichette 2 , Reference Gonzalez-Sarrias, Li and Seeram 3 In this study we demonstrate that an ethyl acetate maple syrup extract decreases oligomerization and aggregation of both β-amyloid (Aβ) and tau peptides in vitro, the two pathological hallmarks of AD.
Methods
Extractions
Maple syrup (Canada No. 3 - Dark; White Meadows Farms, St. Catherine’s, Ontario) was extracted following previously described procedures.Reference Li and Seeram 5 One litre of syrup was first extracted with ethyl acetate (3×500 mL) and dried using sodium sulfate prior to evaporation of the combined fractions in vacuo to yield 0.118 g of a light brown solid (Extract A). Further extraction of the syrup with n-butanol (3×500 mL) yielded a brown solid following drying with sodium sulfate and removal of solvent in vacuo which was then extracted (3×10 mL) to produce a methanol soluble (1.40 g brown oil) and insoluble (2.31 g light brown solid) extracts (Extracts B and C). Previous studies by Li and Seeram, using the same extraction method, have characterized the components of the ethyl acetate and n-butanol extracts of maple syrup.Reference Li and Seeram 4 , Reference Li and Seeram 5 Thirty different compounds including a variety of ligands and polyphenols were identified in the ethyl acetate extract,Reference Li and Seeram 4 while the n-butanol extract contained an additional 23 compounds including lignans, phenolic derivatives, stillbene and coumarins.Reference Li and Seeram 5 Solutions of the extracts were prepared in dimethylsulfoxide (DMSO) in various concentrations for in vitro testing, with tested concentrations of 30, 6 and 1.2 ppm.
β-Amyloid ThT Aggregation Assay
Thioflavin T (ThT; 4-(3,6-dimethyl-1,3-benzothiazol-3-ium-2-yl)-N,N-dimethylaniline chloride) is a benzothiazole dye with a high affinity for proteins containing high β-sheet content used in the visualization of β-amyloid aggregates. Unbound ThT has fluorescence excitation (λex) and emission (λex) wavelengths at 430 nm and 342 nm respectively, which upon binding to aggregated β-amyloid undergoes a characteristic spectral shift (λex=442 nm, λem=482 nm). This spectral shift is used to differentiate bound and unbound ThT. β-Amyloid 1-40 (>95%) was purchased from AnaSpec (Freemont, California) and stored at −80°C. All other reagents were of the highest available purity, purchased from Sigma-Aldrich (Oakville, Ontario), and used without further purification. All water used in the assays was micropore filtered and deionized. β-Amyloid 1-40 (1.0 mg) was pretreated in a 1.5 mL microfuge tube with 1 mL hexafluoro-2-propanol (HFIP), and sonicated for five minutes to disassemble any pre-formed β-amyloid aggregates. Hexafluoro-2-propanol was removed using a stream of argon prior to dissolution of β-amyloid in Tris base (1 mL, 20 mM, pH 10) using vortex and 10 minutes sonication. The solution was then transferred to a 15 mL glass vial and diluted with a further 4.7 mL of the Tris base followed by adjusting to pH 7.4 using concentrated HCl (aq) and filtered using a 0.2 μm syringe filter.
The pretreated β-amyloid was diluted with an equal volume (5.7 mL) of 8 μM ThT in Tris (20 mM, pH 7.4, 300 mM NaCl) and 200 μL aliquots were added to wells of a black polystyrene 96-well plate. 0.4 μL of samples in dimethyl sulfoxide (DMSO) were added to each well. Each sample was performed in triplicate and DMSO alone served as a control to ensure that any observed anti-aggregant activity was accurately attributed to the samples being tested. Plates were covered with clear polystyrene lids and incubated in a Tecan Genios microplate reader at 37°C with fluorescence measurements recorded (λex=450 nm, λem=480 nm) every 15 minutes after first being shaken at high intensity for 15 seconds and then allowed to settle for 10 seconds before each reading.
β-Amyloid Oligomerization Assay
This assay, adapted from LeVine,Reference LeVine 6 was used to determine the ability of compounds to inhibit the oligomerization of biotinylated β-amyloid (1-42) utilizing an enzyme-linked immunosorbent assay, (ELISA) method on a NeutrAvidin-coated plate. An ELISA plate (Costar 9018) was coated with 50 μL of 1 μg/mL NeutrAvidin™ (NA) in 10 mM sodium phosphate buffer, pH 7.5. The plate was sealed with adhesive film and stored at 4°C overnight prior to blocking for two hours at room temperature with 200 μL/well of sodium phosphate buffer (20 mM, 150 mM NaCl, pH 7.5) plus 0.1% v/v Tween 20. Then, 20 μL of a 0.1 mg/mL solution of biotinylated β-amyloid was further diluted with 100 μL HFIP and dried under a stream of nitrogen. Trifluoroacetic acid (TFA, 100 μL) was added to the tube and the sample was dissolved using a vortex mixer prior to drying under a stream of nitrogen. 100 μL of HFIP was added and dried under a nitrogen stream to remove residual TFA. The biotinylated β-amyloid was then dissolved in 870 μL of DMSO. 4.3 μL of test compound dissolved in DMSO was added to each well of a 96-well polypropylene plate (Nunc 267245) and 430 μL of 20 mM sodium phosphate buffer, 150 mM NaCl, pH 7.5). 2 μL of the biotinylated β-amyloid solution was added to each well of a 96-well polypropylene plate (Costar 3365) followed by 100 μL of diluted test compound. The plate was incubated for one hour at room temperature.
The NeutrAvidin-coated plate was warmed to room temperature and the blocking solution removed. 50 μL of the biotinylated β-amyloid and test compound solution was added to each well and the plate was once again sealed and incubated for two hours with shaking at 150 rpm. A plate washer (3×30 seconds, 200 μL/well) with 20 mM Tris-HCl (150 mM, pH 7.5/0.1% Tween 20) was used following incubation. Then, 50 μL of Streptavidin-HRP (1:20,000) in sodium phosphate buffer (20 mM, 150 mM NaCl, pH 7.5 + 0.1% Tween 20) was added and sealed prior to incubation for one hour with shaking at 150 rpm. The plate was once again washed using 20 mM Tris-HCl (150 mM, pH 7.5/0.1% Tween 20) 3X 30 seconds, 200 μL/well. 100 μL of tetramethylbenzidine TMB/H2O2 substrate solution was added to each well. Reaction was stopped after 5-10 minutes with the addition of 100 μL of 1% v/v sulfuric acid prior to reading absorbance at 450 nm in a plate reader.
Tau ThS Aggregation Assay
The thioflavin S (ThS) assay is a fluorescence assay based assay used to visualize the aggregation of tau protein; ThS is a benzothiazole dye with a high affinity for proteins containing high β-sheet content. The assay protocol is analogous to the ThT assay used to examine the aggregation of β-amyloid except for the requirement of an inducer (heparin) to initiate tau aggregation. Full-length human tau, 2N4R isoform, was purchased from rPeptide (Bogart, GA). Each experiment consisted of a single replicate and was repeated four times.
A 4 μM solution of tau in 5 μM ThS in Tris (50 mM, pH 7.4, 0.01 mg/mL heparin, 1 mM DTT, 50 μM NaN3) was prepared fresh for each experiment and 200 μL aliquots were added to wells of a black polystyrene 96-well plate. 0.4 μL of samples in DMSO were added to each well. Each sample was performed in triplicate and DMSO alone served as a control to ensure that any observed anti-aggregant activity was accurately attributed to the samples being tested. Plates were covered with clear polystyrene lids and incubated in a Tecan Genios microplate reader at 37°C with fluorescence measurements recorded (λex=450 nm, λem=480 nm) every 15 minutes.
Results
Three separate extracts of maple syrup were sequentially isolated: Extract A – ethyl acetate soluble; Extract B – n-butanol soluble, methanol insoluble; Extract C – n-butanol soluble, methanol soluble. Each of these three extracts was then evaluated for anti-protein misfolding activity in three separate assays: [i] Aβ anti-oligomerization assay, [ii] Aβ anti-aggregation assay, and [iii] tau anti-aggregation assay. The use of thioflavin T and thioflavin S allowed for the dynamic measurement of aggregation such as lag time, initial rate of beta-sheet formation and equilibrium levels. A clear distinction in activity between the three extracted fractions from maple syrup was noted, with the ethyl acetate soluble fraction (A) demonstrating significant Aβ anti-oligomeric and Aβ anti-aggregant activities as well as the ability to prevent tau aggregation. Extract A was shown to inhibit Aβ aggregation by 47% at 30 ppm, and demonstrated concentration-dependent activity in the ThT assay (Figures 1, 2). Extracts B and C did not exhibit any significant activity, and there was no detectable effect of compound concentration on Aβ aggregation. This trend was also demonstrated in the Aβ oligomerization assay with A demonstrating significant activity, while both Extracts B and C showed no significant difference from the control (p=0.05, two-tailed, Figures 3 and 4). In addition, the thioflavin S assay was employed to determine whether extract A would also prevent aggregation of tau; extract A was shown to inhibit the aggregation of tau by 41% at a concentration of 30 ppm (Figure 5). Extracts B and C had no activity in the ThS aggregation assay.

Figure 1 Thioflavin T assay results. β-Amyloid was incubated with 100μM of each maple syrup extract, A – ethyl acetate soluble; B – butanol soluble, methanol insoluble; C – butanol and methanol soluble. DMSO was used as a control compound.
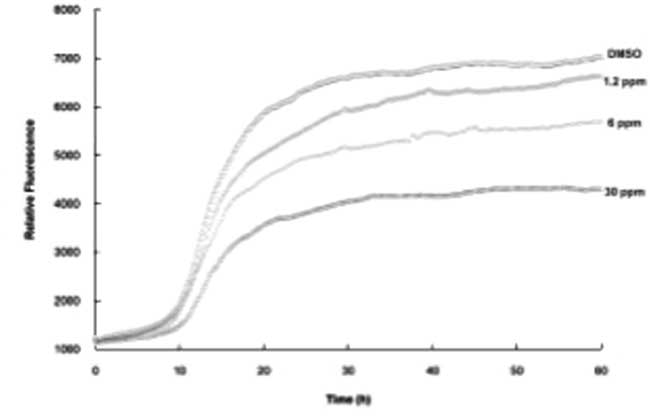
Figure 2 Thioflavin T assay results. β-Amyloid was incubated with varying concentrations of the ethyl acetate extract of maple syurp (C). DMSO was used as a control.

Figure 3 β-Amyloid oligomerization assay results for maple syrup extracts (100μM). Extract A, is shown to cause a significant decrease in oligomer formation as indicated by * (p=0.05). DMSO was the control compound used.

Figure 4 β-Amyloid oligomerization results. β-Amyloid was incubated with 100μM of each maple syrup extract, A – ethyl acetate soluble; B – butanol soluble, methanol insoluble; C – butanol an μd; methanol soluble. DMSO was used as a control compound.

Figure 5 Thioflavin S assay results. β-amyloid was incubated with 100μM of the ethyl acetate soluble extract of maple syrup (A). DMSO was used as a control compound.
Although Extract A has significant anti-protein misfolding activities, it is difficult to identify which exact compound(s) imparts these activities. Li et al. have identified a wide variety of compounds including lignans, polyphenols and a phenylpropanoids within ethyl acetate extracts of maple syrup.Reference Li and Seeram 4 Moreover, it is possible that a single component of the extract is not responsible for the observed biological activities, and that a combination of components leads to the observed results. Nonetheless, the results of this study do indicate that the ethyl acetate extract of maple syrup has significant anti-protein misfolding activity which is within the range of other natural product polyphenols. For example, the IC-50 for inhibition of Aβ oligomerization by maple syrup Extract A was 6.6 ppm, which is comparable to a range of other polyphenols, including resveratrol (red wine extract) and (—)-epigallocatechin-3-gallate (green tea extract), which have IC-50s in the 12-15 μM range. Investigations into the ability of these compounds to cross the blood brain barrier must be performed in order to estimate the required intake of maple syrup needed to have a therapeutic effect along with more advanced in vivo experiments.
Conclusions
This study provides a uniquely Canadian addition to the literature pertaining to the potential anti-Alzheimer’s benefits of natural product polyphenols. Through a series of in vitro experiments we were able to identify the potential ability of an ethyl acetate extract of maple syrup to reduce β-amyloid and tau aggregation, two pathological hallmarks of Alzheimer’s disease. It is still unknown whether the compounds and/or their metabolites are able to cross the blood brain barrier, with more advanced in vivo models being required to further understand the potential activities. While we are currently unable to identify a specific component of the extract responsible for this activity, the antiaggregation properties of maple syrup are analogous to those of other natural products extracted from red wine or green tea. Further work is required to ascertain if maple syrup derived natural products could be a starting point in the design and development of new therapeutics for AD.
Disclosures
Donald Weaver and Cassandra Hawco have nothing to disclose. Marcia Taylor has the following disclosure: Treventis Corp, Director, Salary, Stock options. YanFei Wang has the following disclosure: Treventis Corp, Director, Salary, Stock options.







