Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder with the development of increased frequency and duration of OFF times, unpredictable or sudden OFF, dose failures, and dyskinesias over time which are unresponsive to standard oral medications. These affect patients’ independence and quality of life (1–3). In Canada, two therapies can be offered in advancing stages: deep brain stimulation (DBS) and levodopa-carbidopa intestinal gel (LCIG). Reference Giugni and Okun1–Reference Antonini, Moro, Godeiro and Reichmann3
LCIG has gained popularity as a treatment option as it provides a stable level of drug delivery. It has two-fold pharmacokinetic benefit over oral levodopa treatment: delivery in the continuous manner leads to stable rather than pulsatile plasma levels of levodopa and direct delivery at the site of absorption in the jejunum circumvents erratic gastric absorption and increases bioavailability. Reference Kurlan, Rubin, Miller, Rivera-Calimlim, Clarke and Shoulson4
LCIG has been used for the treatment of PD in Europe for almost two decades since its approval in Sweden in 2004. Reference Wirfeldt, Odin and Nyholm5 In Canada, Health Canada approval was obtained in 2014. The short-term benefit (7–28 weeks) and safety profile of LCIG are well established Reference Othman and Dutta6–Reference Nyholm, Askmark and Gomes-Trolin9 but long-term experience is limited with majority of the data coming from European centers. Reference Devos, Agid and al Khedr10–Reference Valldeoriola, Grandas and Santos-García21 Despite the benefits, LCIG is a relatively under-utilized therapy compared to DBS in North America. Reference Rasameesoraj22
In Edmonton, LCIG has been available since 2011 (originally under research protocol) and is performed in a multidisciplinary setting composed of neurology, gastroenterology, psychiatry, physical and occupational therapy, and specialized nurses. As LCIG is a complex and costly long-term treatment, the aim of our study was to analyze long-term experience in the clinical management of PD patients with LCIG therapy over an 11-year period in a Canadian setting, highlighting the elements which we believe are essential to providing LCIG as a reliable therapeutic option.
Methods
Protocol for patient selection for LCIG: Patient selection involved initial screening by the neurologist with detailed assessment of motor fluctuations, hours with dyskinesias, levodopa responsiveness (including UPDRS-III), cognition (MoCA), psychiatric evaluation (if needed), social supports, and functional status. Then, the patient was seen by the gastroenterologist to assess for PEG-J insertion. Inclusion and exclusion criteria are summarized in Table 1.
Table 1: Inclusion and exclusion criteria of patient selection for LCIG therapy

Admission and titration: During initial 5 years of the study period, nasojejunal (NJ) tube was placed for 1–2 days during in-patient admission to the clinical investigation unit with LCIG titration to an effective dose by the neurologist and nurse. Patients and caregivers were taught to use and manage the LCIG pump. With demonstrated benefit and patient comfort, PEG-J was inserted followed by 1–2 days of titration in hospital. From 2017 onwards, patients, who were good candidates, went directly for PEG-J insertion followed by in-hospital titration for 1–2 days. However, patients who were unsure of the benefit or reluctant to directly go for PEG-J insertion still had NJ trial phase.
Clinic visits: Patients were seen at the Movement Disorders Clinic every 2–3 months in the first year after LCIG initiation. Afterward, appointments were decreased to 3–4 months.
Data collection: Consecutive patients on LCIG therapy at our center between 2011 and 2022 were included. The last patient in our cohort initiated on LCIG therapy in 2021 and the follow-up data were collected for all the patients until September 2022. This is an ambidirectional cohort study with retrospective chart review performed from 2011–2016 while data were collected prospectively from 2017 onwards. Four patients initially entered under a study protocol but then transferred to clinical care from 2014 onwards. All data presented were obtained from clinical charts. Following information was extracted:
-
1. UPDRS-III scores (motor subscale; pre- and post-LCIG every 6 months) *
-
2. Montreal Cognitive Assessment (MoCA) scores (pre- and post-LCIG annually) *
(Done prior to need for registration)
-
1. Hours with dyskinesia and OFF periods
-
2. LCIG pump and PEG-J complications and the treatments
-
3. Information about the patient and caregiver experience, including the pros and cons of LCIG therapy, collected in 2018–2020 by MH
-
4. Nursing hours and time spent attending to patient telephone calls related to LCIG were collected from 2017–2022 during and in between clinic visits.
* During COVID-19 pandemic from 2020–2021, majority of the clinic visits were virtual, and hence, UPDRS-III and MoCA score data are not available for many of the patients.
This study received University of Alberta Human Research Ethics Board approval. (Approval number – Pro00086619)
Statistical Analysis
Clinical data were analyzed using descriptive statistics. Numbers and percentages were used for describing the categorical data while mean with standard deviation and median with range for expressing the continuous data. Student’s paired t-test was used to compare the baseline and the follow-up values to analyze the efficacy parameters. All p values reported are two-tailed, and P value < 0.05 was considered statistically significant.
Results
Thirty-three advanced PD patients were enrolled over 2011–2021 for LCIG with a mean age of 68.50 ± 7.49 (mean ± standard deviation) years. Sixteen patients had NJ tube trial phase followed by PEG-J insertion while 17 patients went directly for PEG-J. Demographic and clinical characteristics of the patients are shown in Table 2. Of 33 patients, 22 were males and 11 females with no significant differences in their baseline characteristics.
Table 2: Baseline demographic and clinical characteristics of 33 patients treated with LCIG (22 males and 11 females)
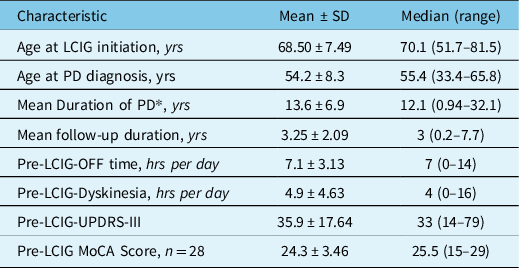
* At time of LCIG initiation.
The mean duration of LCIG therapy for whole cohort was 3.25 ± 2.09 years. Two patients have remained on LCIG for more than 7 years. Eleven (33.3%) patients died during the study period after 3.21 ± 2.11 years from LCIG initiation. Causes of death are listed in Figure 1 with only one death related to LCIG therapy due to upper gastrointestinal bleeding from gastric ulceration caused by knotted PEG-J tube as shown in Figure 2. Nine patients (27.2%) discontinued the treatment after 3.7 ± 2.18 years including seven patients due to lack of improved efficacy over oral dopaminergic therapy in terms of motor fluctuations and/or dyskinesia/ dystonia (2 of these transitioned to DBS) and two patients due to worsening of cognition. Fourteen (42.4%) patients remain on LCIG with mean follow-up of 3.27 ± 2.0 years. The average period of daytime infusion ranged between 11–15 hours in 27 patients while six patients transitioned to 24 hours of infusion due to nocturnal bradykinesia and/or rigidity.
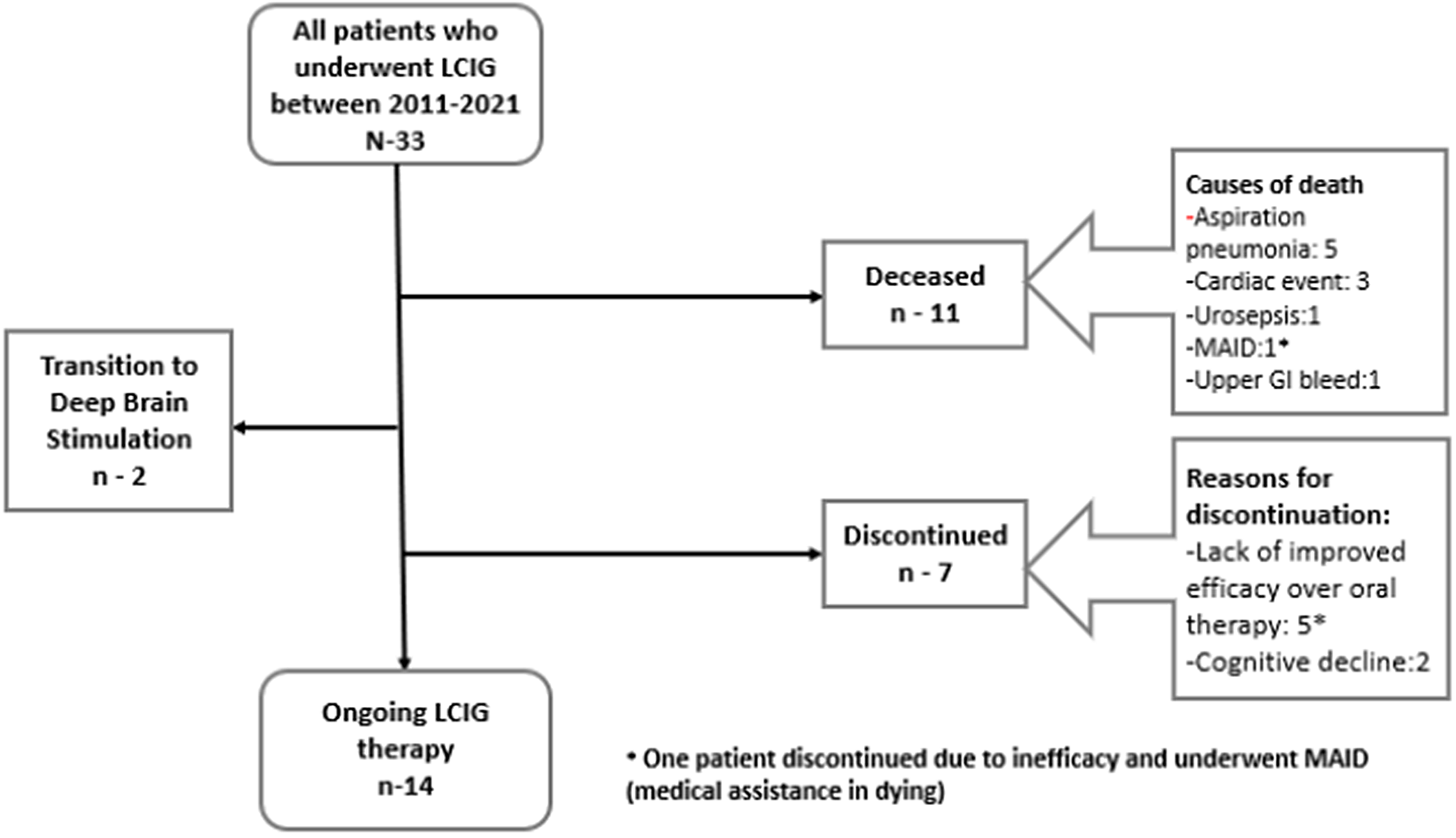
Figure 1: Flowchart of patient outcomes.

Figure 2: Endoscopic view of PEG-J tube knot (indicated by arrow) as one of the complications seen in one of our patients.
Effect on PD Symptoms
All patients showed improvement in motor fluctuations as shown in Figure 3a with significant reduction in the mean daily OFF time from baseline (pre-LCIG; 7.1 ± 3.13 hours) up to 5 years (3.3 ± 2.31 hours; -53.5%; p < 0.048). The number of hours with dyskinesias improved significantly at 1 year (2.1 ± 3.13 hours; p < 0.005) as compared to baseline (pre-LCIG; 4.9 ± 4.63 hours). However, it increased after 1 year to reach baseline by 3 years (5.0 ± 5.4 hours) and then remained stable until 5 years (Fig. 3b). The UPDRS-III scores improved by −9.4 points (p value – 0.002) at 1 year and worsened after 4 years (Fig. 4a, b). Cognitive function evaluated with MoCA remained stable (p - 0.14) with mean score of 26 at baseline (n - 28) and 23 at 3 years (n - 9) before declining significantly (p - 0.003) between 4-− years (n - 7, mean - 19).
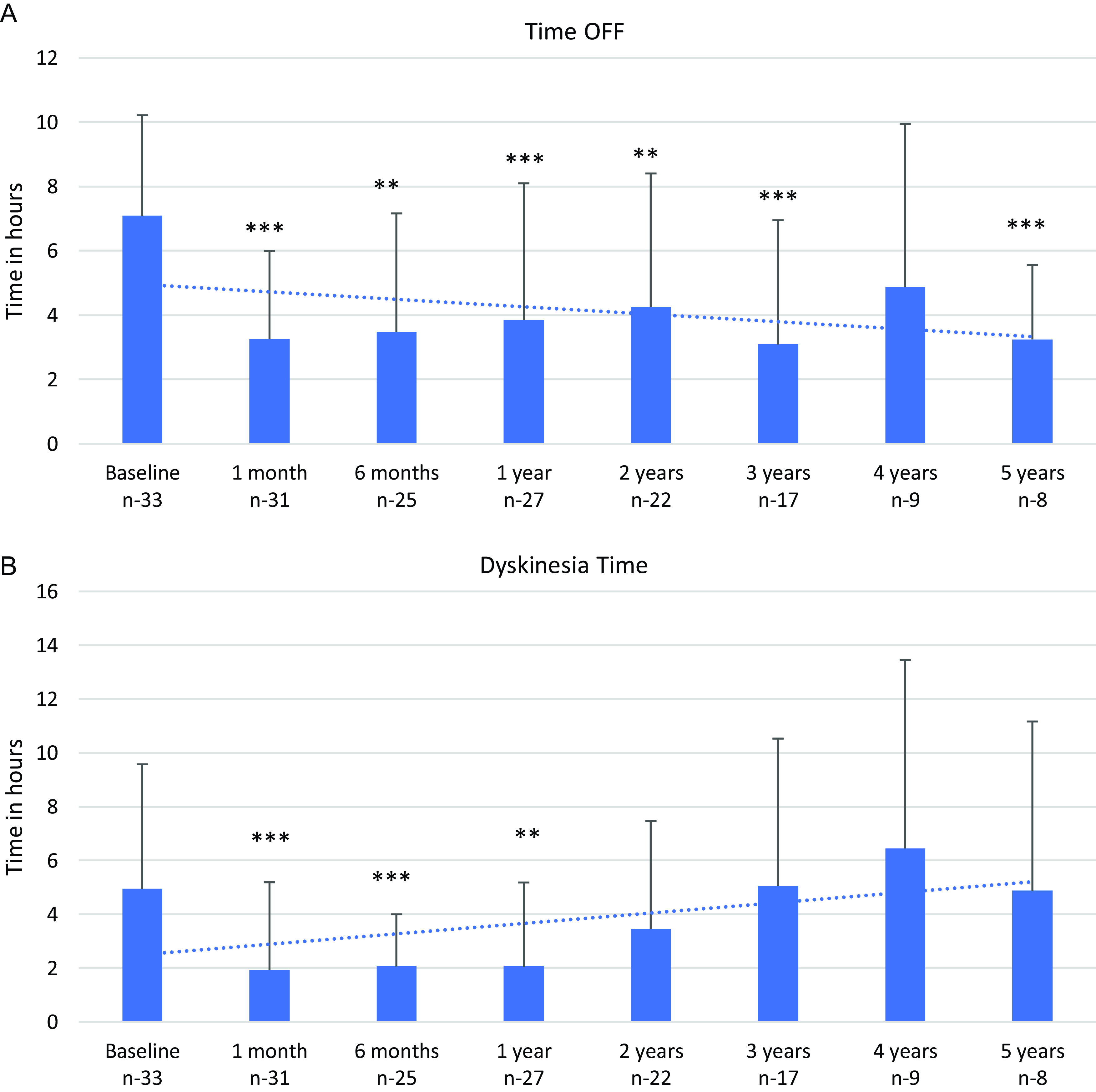
Figure 3: a : Time off from baseline to 5 years. b : Duration with dyskinesias from baseline to 5 years. Error bar indicated SD. Asterisks indicate statistical significance compared to baseline in a paired t-test at the P < 0.01 (**) and P < 0.001 (***) levels.

Figure 4: a : Serial UPDRS-III motor scores from baseline to 5 years. b : Changes in LCIG levodopa equivalent daily dosing (LEDD) in milligrams with time. Asterisks indicate statistical significance compared to baseline in a paired t-test at the P < 0.01 (**).
Pharmacological Therapy
The mean LEDD showed a rising trend from 1340 ± 526 mg/day at baseline to 1665 ± 806 mg/day at 2 years (p- 0.005). The requirement decreased subsequently to fall below the mean baseline LEDD from 4 years onwards (n – 10, 1300 ± 824.3 mg/day, p - 0.2) as shown in Figure 4b. At this time, MoCA scores were worsening with development of hallucinations.
Complications
Complications were encountered in 28 patients (84.8%) during the study period and are listed in Table 3. The most common complication was PEG-J dislodgement (n - 18) with either retrograde or antegrade migration of the J tube (0−3 dislodgement/patient/year). There were 87 PEG-J replacements in 18 patients over the study period (0−4 replacements/patient/year) due to tube dislodgement, obstruction, kinking, and/or tube malfunction. Tube replacement is not done electively at our site. Stoma site infection was seen in 16 patients and easily treated with antibiotics. Fungi including candida and yeast were the most common organism identified on culture followed by bacteria, staphylococcus, streptococcus, and Ekinella. Serious adverse events were rare, affecting four patients, and included abdominal wall erosion, abdominal wall dehiscence, and buried bumper syndrome. PEG site-related gastric ulceration causing upper gastrointestinal bleeding resulted in death in one patient.
Table 3: Total number of complications in our cohort from 2011 to 2022
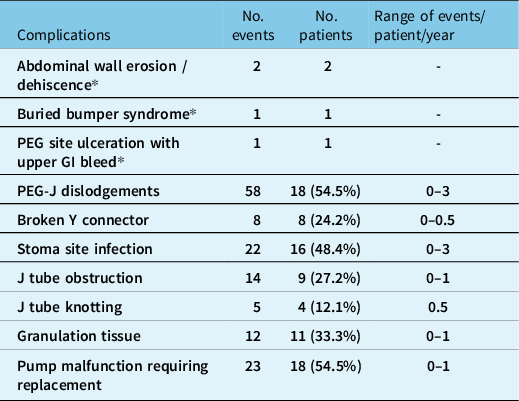
* Serious adverse events requiring hospitalization.
Neuropsychiatric complications are summarized in Table 4. The most common complication was visual hallucinations seen in 12 (36%) patients developing over 1−3 years after LCIG initiation. Of these, cognitive impairment occurred in seven patients over next 1−2 years. Dopamine dysregulation syndrome, punding, and psychosis were seen in 6% each. These complications were treated by decreasing the dose of LCIG and/or addition of psychiatric medications such as quetiapine and/or cholinesterase inhibitors.
Table 4: Neuropsychiatric complications

* 7 patients with visual hallucinations developed cognitive impairment over next 1–2 years.
Nursing time Requirement
Significant tasks that need to be completed with average nursing time requirements per patient are listed in Table 5. At our center, one 0.5 FTE nurse is devoted to the LCIG program and dedicates an average of 4 hours for screening, education, and securing funding. During in-patient admission for PEG-J insertion and titration, nurses are involved for 8–16 hours per day. Post-discharge nursing time averaged 22 hours per patient per year dealing with complications, pump settings, and securing yearly funding and delivery.
Table 5: List of tasks completed/arranged by nurse for each patient starting LCIG and average nursing time requirement

Patient and Caregiver Reported Benefits
Patients and their caregivers reported benefits with LCIG being increased ON times, ability to do fine adjustments with improved control of dosing, and freedom from frequent intake of oral levodopa-carbidopa. Cons reported by patients included requirement for frequent battery changes (1−3 weeks), embarrassing alarms going off in public setting, discomfort sleeping with PEG-J, increase worry while traveling, and tube dislodgement requiring hospital visits.
All patients starting on LCIG had caregivers. Eight patients transitioned to long-term care over the study period, and nursing staff at long-term care were trained to administer LCIG with good results.
Discussion
This ambidirectional cohort study from Canada reports on 11 years of experience in managing advanced PD patients who underwent LCIG therapy in a single multidisciplinary clinical setting. Our results confirm efficacy of LCIG therapy in improving motor fluctuations, motor symptoms, and stabilizing dyskinesias. Overall patient satisfaction was high with some patients continuing LCIG up to 7 years. Lack of improved efficacy over oral dopaminergic therapy and development of dementia are the main reasons for discontinuation. Complications although most are minor require a dedicated experienced team for management. Caregiver support is important during independent living, but good care can be provided in a long-term care setting.
Two previous multicenter observational studies from Europe with 73 and 375 patients, respectively, on LCIG therapy reported 37.1% and 31.6 % reduction in the mean daily OFF time at 2 years. Reference Antonini, Odin and Opiano12,Reference Antonini, Poewe and Chaudhuri15 A marked 81.6% improvement in motor fluctuation at 2 years was seen in another single-center prospective observational study from Spain (23). Reference De Fabregues, Dot and Abu-Suboh23 Two studies from Italy reported 50% reduction in the mean daily OFF period at 3 years, Reference Zibetti, Merola and Ricchi11,Reference Lopiano, Modugno and Marano19 similar to our results. Only two studies have reported improvement in the OFF time over follow-up of 4−5 years Reference De Fabregues, Dot and Abu-Suboh23,Reference Antonini, Bondiolotti, Natuzzi and Bareggi24 with 68% and 83% reduction, respectively. A recent multinational study including 262 patients with a mean follow-up of 4.1 years reported improvement in the motor fluctuations with decrease of 4 hours in the mean daily OFF time from baseline. Reference Fernandez, Boyd and Fung25 Our study shows similar benefit.
A 7-year follow-up study showed 30% reduction in dyskinesia duration. Reference Zibetti, Merola and Artusi26 In another study with 10 years of follow-up with 37 patients, dyskinesias reduced in 16.1% of patients but remained stable in 83.8%. Reference De Fabregues, Dot and Abu-Suboh23 Dyskinesia duration remained unchanged in another recent study with a mean follow-up of 4.1 years. Reference Fernandez, Boyd and Fung25 Our study showed significant reduction in dyskinesia duration at 1 year followed by increase in duration till 3 years, which then remained stable until 5 years.
Very few long-term follow-up studies reporting serial changes in the mean UPDRS-III scores are available with mixed results. Reference Antonini, Odin and Opiano12,Reference Zibetti, Merola and Artusi26,Reference Fasano, Ricciardi, Lena, Bentivoglio and Modugno27,Reference Fernandez, Standaert and Hauser28 In a multicenter study from Europe with 73 patients and a mean follow-up of 2 years, a significant improvement by -3 points was seen at 6 months, which continued for 1 year. Reference Antonini, Odin and Opiano12 In another open-label study with 28 patients, motor scores improved by −3.5 points at 1 week and the benefit was persistent till 60 weeks. Reference Standaert, Rodriguez and Slevin29 In our study, UPDRS-III scores improved significantly at 1 year by −9.4 points from baseline with persistence until 4 years. However, the motor symptoms as evaluated by UPDRS-III score as well as cognition as assessed by MoCA score worsened after 4 years, which correlates with the decrease in the total LEDD from 4 years onwards. We hypothesize that progression in PD led to worsening of cognition, visual hallucinations, and psychosis. Treatment by decreasing the continuous rate of LCIG can be helpful, but results in worsening of the motor scores.
Zibetti et al reported worsening of the MMSE scores from 24.7 to 15.6 in 41% of the patients at end of 3 years of follow-up on LCIG therapy. Reference Zibetti, Merola and Ricchi11 In contrast, another 5-year study with 20 patients reported no significant change in MMSE scores (29.3 vs 26.6, p value - 0.3) at the end of follow-up period (30). In the present study, MoCA remained stable for almost 3 years and then worsened significantly between 4 and 5 years, leading to discontinuation of LCIG in two patients. The differing results in these studies are due to different time lengths of follow-up and an older cohort being treated with LCIG in our study and the study by Zibetti et al. Reference Zibetti, Merola and Ricchi11
The complications and the safety profile reported in this study are consistent with the previous studies with device-related issues being most common and low incidence of SAEs. In a systematic review, LCIG discontinuations due to device-related issues were reported in<5% (31) but none in our study. Device-related complications occurred in 84.8% of the patients in our study, which is similar to 93% observed in another study with mean follow-up of 4.1 years. Reference Fernandez, Boyd and Fung25 The number of patients with PEG-J replacements varied between 60%–90% in previous long-term studies with follow-up ranging from 4 to 10 years Reference De Fabregues, Dot and Abu-Suboh23,Reference Fernandez, Boyd and Fung25 , which is slightly more as compared to 54.5% in our study.
As can be seen, LCIG is a safe and efficacious therapy for advanced PD for up to 7 years. Several criteria need to be in place. First, appropriate patient selection is the key element of successful LCIG program. Reference Rasameesoraj22 Secondly, a multidisciplinary team is needed for the care of these complex patients and the coordination of an LCIG program. An effective collaboration between the neurologist and gastroenterologist having expertise in specialized set of PEG-J tubes is vital. A specialized nurse is another important member of the team, for preparing patients to start LCIG, participating in titration, troubleshooting, and dealing with expected or unexpected issues. Identifying a reliable caregiver is equally important as patients may not be able to manage the pump or complications.
Recently, our protocol has changed with PEG-J insertion done as day surgery with patients coming after 2–3 weeks for out-patient titration for 3 days consistent with other centers in Canada. Reference A.L.Ghamdi, Rumman and Liu32 However, this does not decrease nursing time requirements with respect to education and titration or multidisciplinary management with caregiver support.
Other studies as well as systemic reviews have further shown that the benefits of LCIG extended beyond the motor symptoms of PD and include improved sleep/fatigue, attention/memory, gastrointestinal tract and urinary symptoms, and sexual function with improved quality of life. Reference Antonini, Odin and Pahwa31 Although development of tolerance was a concern early on for patients receiving LCIG for extended periods, Reference Standaert, Rodriguez and Slevin29 several reports including the present study (six patients on 24- hours of infusion) have shown this is not the case and benefit is seen for nocturnal akinesia and sleep disruption. Reference Ricciardi and Bove33–Reference Kovács, Szász and Vela-Desojo35
The main strategy for the treatment of advanced and complex PD in Canada is deep brain stimulation (DBS) with rate of DBS surgery being ten per million population per year. Reference Honey, Malhotra and Tamber36 Despite the demonstrated benefit of LCIG, DBS is performed at a much higher rate. For example, in the USA, between 2015 and 2017, 900 patients received LCIG as compared to 13,000 receiving DBS . Reference Rasameesoraj22 Similarly, approximately 40 patients received DBS in northern Alberta (Edmonton) from 2015 to 2016 as compared to only seven patients who received LCIG during same period in the present study. Reference Honey, Malhotra and Tamber36 While there are no head-to-head randomized controlled trials comparing these two advance therapies, a recent metanalysis have shown LCIG to be comparable to STN DBS in term of improvement in motor symptoms but less efficacious with respect to improvement in dyskinesias and motor fluctuations. Reference Liu, Bao and Liu37 DBS is the treatment of choice in younger patients with preserved cognition, but LCIG is the reasonable option for those who are ineligible or do not wish for invasive neurosurgery. Reference Dijk, Espay, Katzenschlager and de Bie38
Conclusion
Our study confirmed long-term efficacy of LCIG in treatment of advanced PD as evidenced by improvement in motor scores, reduced OFF times, and stable dyskinesias over 5-year follow-up.
Complications are frequent but generally mild and necessitate a well-trained multidisciplinary team for management.
Acknowledgements
We acknowledge all patients and families who agreed to participate in this study.
Author contribution
Research Project Conception: OS.
Research project organization, Statistical analysis design: CV, AS, MH, AL, OS.
Data collection: CV, MH, GT, JM, AS, AL, OS.
Statistical analysis: CV.
Writing of the draft: CV, MH, OS.
Review and critique: CV, MH, GT, JM, AS, AL, OS.
Financial support
None.
Competing interests
OS and AS have research grants from Wave Life Sciences, Roche, CHDI University Hospital Foundation, are on the scientific advisory boards for AbbVie and Sunovion, are on Data Safety Monitoring Committee for Alexion, and receive royalties from UpToDate.











