Introduction
Chronic constipation is a common gastrointestinal disorder affecting 16% of adults with direct medical costs over $230 million annually Reference Bharucha and Wald1 in addition to lost earnings from reduced work productivity. Reference Heidelbaugh, Stelwagon, Miller, Shea and Chey2 Criteria for chronic constipation include the presence of fewer than three bowel movements per week, the strained passage of bowel movements, and/or lumpy or hard stools in over a quarter of defecations in the past 3 months. Reference Mearin, Lacy and Chang3 Untreated chronic constipation, particularly in the middle-aged and elderly, Reference Gallagher and O’Mahony4 can result in bowel obstruction, stercoral ulcers, fecal impaction, and anal fissures. 5 For these reasons, 72% of patients with chronic constipation use laxatives of various types to relieve their symptoms. Reference Werth and Christopher6,Reference Johanson and Kralstein7 Research has also suggested that constipation affects sleep Reference Duboc, Coffin and Siproudhis8 and may be associated with functional impairment and reduced quality of life as a consequence of sleep disruption. Reference Ohkubo, Yoshihara and Misawa9–Reference Wald, Scarpignato and Kamm11
Prior literature has demonstrated that constipation may be associated with poorer sleep continuity. Constipation was linked to difficulty falling asleep, Reference Gonzalez Canete, Pena D.’ardaillon, Candia Johns and Duran Aguero12 changes in total sleep time, Reference Bouchoucha, Mary, Bon, Bejou, Airinei and Benamouzig13–Reference Shapiro, Bradshaw and Landes16 and overall reduced sleep quality Reference Chen, Bair and Chang17 (Table 1). However, these observational studies, except for one, Reference Shapiro, Bradshaw and Landes16 relied on subjective questionnaires to assess sleep continuity in constipated patients. Past studies have also indicated that constipation may be linked with insomnia. Constipation was associated with waking up more than once a night Reference Cremonini, Camilleri, Zinsmeister, Herrick, Beebe and Talley18 and greater insomnia severity. Reference Bouchoucha, Mary, Bon, Bejou, Airinei and Benamouzig13,Reference Ueki, Nagai and Mizukami14 Table 1 provides an overview of prior studies that have examined the relationship between sleep continuity (or insomnia) and constipation. Reference Gonzalez Canete, Pena D.’ardaillon, Candia Johns and Duran Aguero12–Reference Cremonini, Camilleri, Zinsmeister, Herrick, Beebe and Talley18
Table 1: Observational studies on constipation and sleep quality

* Unless otherwise specified.
While subjective reports of sleep continuity and insomnia have been linked to the presence of constipation particularly in the middle-aged and elderly, the impact of constipation on objective metrics of sleep continuity has remained underexplored. Thus, the primary purpose of this study was to investigate the association of laxative use and laxative subtypes (markers of constipation) on objective markers of sleep continuity in a middle-aged/elderly population (≥40 years old). Secondarily, we aimed to explore whether laxative use was associated with self-reported insomnia symptoms.
Methods
Ethics
This study was approved by the Sunnybrook Research Ethics Board (Study ID 3095: Examining the link between clinical and physiological sleep data and health-related outcomes) for a cross-sectional retrospective analysis of the polysomnographic and clinical data examined in this study.
Study Population and Measures
We collected the sleep and medication data of all patients who completed diagnostic overnight polysomnography at Sunnybrook Health Sciences Centre between 2010 and 2015. Level I, technologist-monitored in-hospital polysomnography (Compumedics Neuroscan, Australia) was performed as previously described Reference Boulos, Murray and Muir19 and scored according to the 2007 American Academy of Sleep Medicine criteria. Reference Iber, Chesson and Quan20 The presence of self-reported insomnia was obtained through a sleep history questionnaire with the following question: “1. Do you have any of the following medical issues? Check all that apply” (insomnia is one of the options). Sleep-related outcomes of interest included sleep onset latency (SOL: time from full wakefulness to sleep onset), sleep efficiency (SE: proportion of total time in bed spent asleep), wake time after sleep onset (WASO: length of periods of wakefulness occurring after sleep onset), total sleep time (TST), and arousal index (AI: number of arousals per hour), as previously described Reference Boulos, Jairam, Kendzerska, Im, Mekhael and Murray21 . Demographic information and medical comorbidities were obtained from questionnaires filled out by patients during the night of their sleep study. Medication logs were collected by a sleep technologist on the night of the sleep study. A graduate student (YSC) subsequently coded medications to identify patients using laxatives of all types including stool softeners, osmotic laxatives, and stimulant laxatives. Laxative use was considered a proxy for chronic constipation since upwards of 72% of patients with chronic constipation use them as treatment. Reference Werth and Christopher6,Reference Johanson and Kralstein7
Risk Factors and Confounders
As observed in previous studies, Reference Peppas, Alexiou, Mourtzoukou and Falagas22–Reference Wald24 age, sex, and body mass index (BMI) were chosen for inclusion as adjustable variables in regression analyses due to their impact on constipation and independent association with sleep continuity Reference Boulos, Jairam, Kendzerska, Im, Mekhael and Murray21 . A literature review was conducted to identify relevant co-morbidities with a significant relationship with chronic constipation from at least one published study; these included stroke, Reference Peppas, Alexiou, Mourtzoukou and Falagas22,Reference Winge, Rasmussen and Werdelin25 diabetes, Reference Peppas, Alexiou, Mourtzoukou and Falagas22,Reference Vinik, Maser, Mitchell and Freeman26 Parkinson’s Disease, Reference Peppas, Alexiou, Mourtzoukou and Falagas22,Reference Ueki and Otsuka27, and use of opioids. Reference Talley, Jones, Nuyts and Dubois28,Reference Camilleri29
Statistical Analyses
For our descriptive statistics, frequency counts were computed for categorical variables. Means and standard deviations (SDs) were calculated for normally distributed continuous variables. For non-normally distributed continuous variables and ordinal data, we calculated the median and interquartile range.
To explore the relationship between laxative use and various objective sleep metrics (TST, SE, SOL, WASO, and AI), we constructed multivariable linear regression models. In the first minimally adjusted models, demographic variables such as age, sex, BMI, and laxative use (all types, stool softener, osmotic, or stimulant) were included in our analyses. In the second fully adjusted models, we included age, sex, BMI, and laxative use as well as clinically relevant comorbidities such as prior stroke, diabetes, Parkinson’s Disease, and opioid use. All variables were assessed for multi-collinearity (defined as a variance inflation factor > 2.5) before the construction of each model. Reference Johnston, Jones and Manley30 All fully adjusted models controlled for the effect of total recording time due to its influence on sleep continuity variables. Reference Maurer, Espie and Omlin31 To relax the assumption of linearity, all continuous variables were modeled as restricted cubic splines with three knots. Reference Harrell32 As recommended, knots were placed at the 10th, 50th, and 90th percentiles of each predictor. Reference Harrell32 Homoscedasticity was visually assessed using the residuals versus fitted values plot. Normality was visually assessed using a residual Q-Q plot; however, no outcome transformations were applied as such transformations can bias model estimates, and models constructed with a large sample size (i.e. where the number of observations per parameter is >10) are generally robust to the normality assumption. Reference Schmidt and Finan33
In our secondary analyses, multivariable logistic regression models were used to explore the relationship between laxative use and insomnia. The first minimally adjusted models included the variables of age, sex, BMI as well as laxative use (all types, stool softener, osmotic, or stimulant). The second fully adjusted models included the variables of prior stroke, diabetes, Parkinson’s Disease, and opioid use in addition to age, sex, BMI, and laxative use. In addition, all fully adjusted models controlled for the effect of total recording time due to its influence on markers of sleep continuity. Reference Maurer, Espie and Omlin31 All continuous variables were modeled as restricted cubic splines with three knots placed at the 10th, 50th, and 90th percentiles of each predictor. Reference Harrell32
In addition, we performed a post hoc sensitivity analysis excluding patients using multiple types of laxatives using multivariable linear regression models (detailed methods and results are available in the supplementary data).
Statistical significance was set to p < 0.05. All data analyses were performed in R (version 3.6.1) using the “rms” package. Model validation was performed using the “0.632 Bootstrap” method to assess the overfitting of the models.
Results
Study Population
A flowchart of the study population is shown in Figure 1. In this study, we cross-sectionally evaluated 2946 patients who completed diagnostic in-laboratory polysomnography (mean age = 60.5 ± 12.0 years; 48.3% male). The use of laxative medication of any type was reported by 3.5% of patients (103/2946; mean age = 71.6 ± 10.6 years; 46.6% male). Of all 103 patients using laxatives, 64 patients were using stool softeners, 17 were using osmotic laxatives, and 45 were using stimulant laxatives (23 patients were using multiple laxative types). Insomnia was reported in 597 patients (20.3% of total sample); 31 patients reporting insomnia were also using laxatives (30.1%; 13 stool softener users, 6 osmotic laxative users, 17 stimulant laxative users, and 5 using multiple laxatives concurrently). The clinical characteristics of the study population are reported in Table 2.
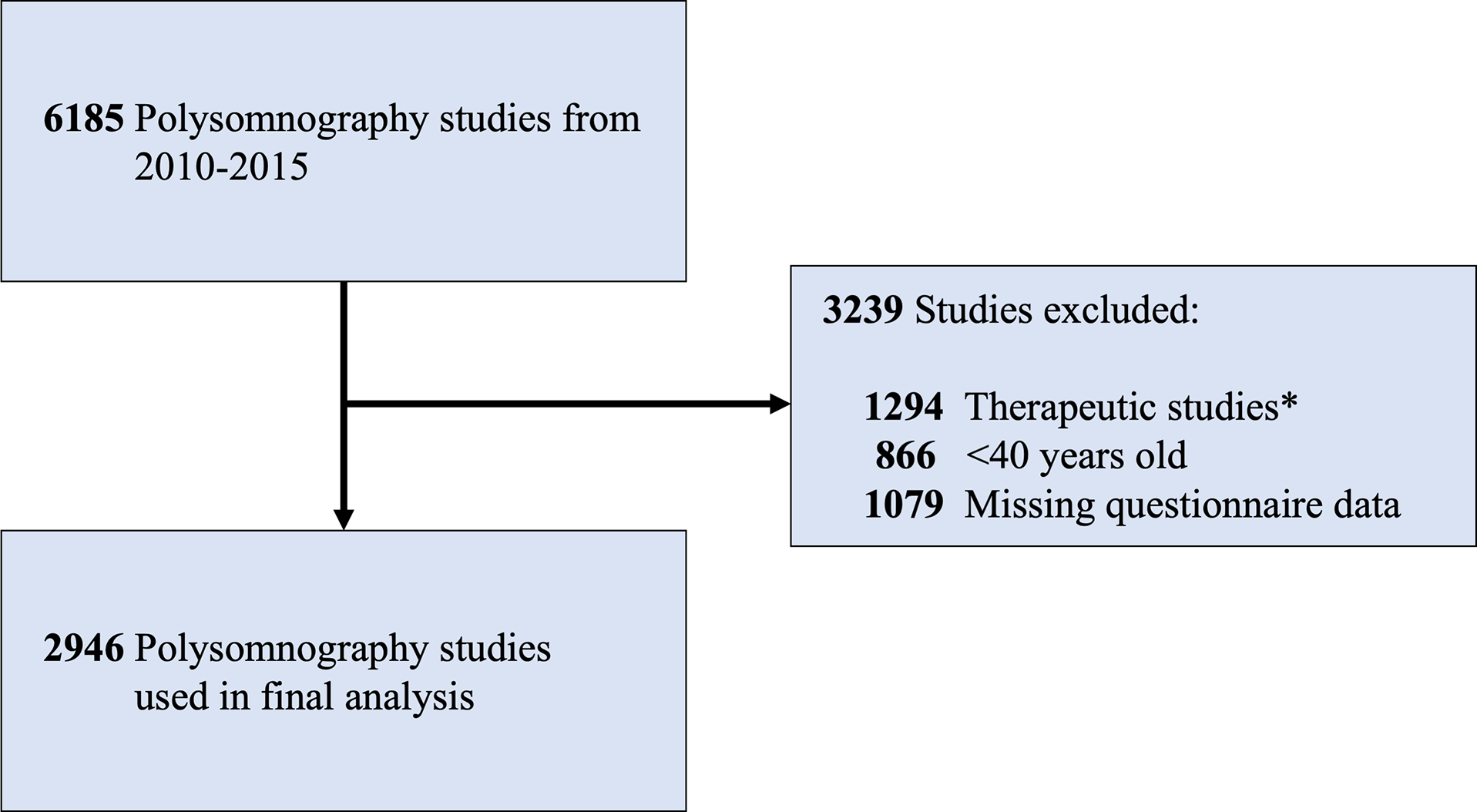
Figure 1: Flowchart of the study population. *Therapeutic studies included continuous or bilevel positive or expiratory airway pressure titrations, split-night studies, and adaptive servo-ventilation studies.
Table 2: Clinical characteristics of the study population according to the use of laxatives

N = 2946, unless otherwise specified
REM: Rapid eye movement.
a Student’s t-test.
b Chi-square test.
Linear Regression Analysis: Laxative Use and Objective Sleep Metrics
In our fully adjusted model, laxative users (all types) had 7.1% lower sleep efficiency (p < 0.001), 25.5-minute greater wake after sleep onset (p < 0.001), and 29.4-minute lower total sleep time (p < 0.001) than non-laxative users. Stool softener users had 6.9% lower sleep efficiency (p = 0.001), 26.3-minute greater wake after sleep onset (p = 0.001), and 28.8-minute lower total sleep time (p < 0.001) than those not using stool softeners. Stimulant laxative users had 5.7% lower sleep efficiency (p = 0.023), 18.3-minute greater wake after sleep onset (p = 0.041), and 23.2-minute lower total sleep time (p = 0.027) than those not using stimulant laxatives. Osmotic laxative use was not significantly associated with changes in sleep metrics in our fully adjusted models. Similar results were also seen in our minimally adjusted models (Table 3).
Table 3: Linear regression models examining the association between laxatives (and subtypes) and sleep metrics while controlling for the impact of various covariates

In our minimally adjusted model, covariates were age, sex, BMI, and laxative use. In our fully adjusted model, covariates were age, sex, BMI, diabetes, stroke, Parkinson’s Disease, opioid use, total recording time, and laxative use. p < 0.05 (bolded).
Logistic Regression Analysis: Laxative Users and Insomnia Symptoms
In both our minimally and fully adjusted models, laxative users (of all types) (p = 0.024) and stimulant laxative users (p = 0.006) were at greater odds of reporting insomnia symptoms than non-laxative users. Stool softener and osmotic laxative users had no statistically significant association with insomnia symptoms (Table 4).
Table 4: Logistic regression analyses on the impact of laxatives and subtypes on insomnia symptoms controlling for the impact of various covariates

In our minimally adjusted model, covariates were age, sex, and BMI. In our fully adjusted model, covariates were age, sex, BMI, diabetes, stroke, Parkinson’s Disease, and opioid use. p < 0.05 (bolded).
Discussion
The main purpose of this study was to ascertain any changes in objective sleep metrics (obtained through in-laboratory polysomnography) with laxative use and to secondarily assess the association of laxative use with self-reported insomnia symptoms in a population over 40 years of age. In both our minimally and fully adjusted models, the use of laxative subtypes was significantly associated with worse sleep continuity, specifically with lower sleep efficiency and total sleep time, as well as greater wake after sleep onset. In addition, in both our minimally and fully adjusted models, laxative users (all types) and stimulant laxative users were at significantly greater odds of reporting the presence of insomnia symptoms.
Our study was unique because we used objective sleep metrics to assess sleep continuity in a relatively large study population compared to prior research. Laxative users (all types), stool softener users, and stimulant laxative users were found to have significantly worse objective sleep continuity in both our minimally and fully adjusted linear regression models. Using laxative use as a proxy for constipation, our results align with past research. Cremonini et al demonstrated that reported trouble staying asleep was more likely in the presence of constipation in a sample of middle-aged adults. Reference Cremonini, Camilleri, Zinsmeister, Herrick, Beebe and Talley18 Cañete et al demonstrated that elderly patients with constipation had greater difficulty falling asleep and reported changes in total sleep time relative to healthy patients. Reference Gonzalez Canete, Pena D.’ardaillon, Candia Johns and Duran Aguero12 Adejumo et al. found increased odds of constipation given shortened sleep duration (<7 hours) based on health surveys. Reference Adejumo, Kuo and Staller15 Similarly, Shapiro et al. demonstrated that there was reduced total sleep time on constipated days relative to normal days based on Fitbit data in a middle-aged population. Reference Shapiro, Bradshaw and Landes16 Majority of these studies relied on self-reported constipation in contrast to ours which relied on inferred constipation based on laxative use.
In our logistic regression analyses, users of all laxative types and users of stimulant laxatives were found to have greater odds of reporting insomnia symptoms than non-users, and this finding persisted with our fully adjusted model. Similarly, past research has indicated an association between insomnia and constipation. Using the Pittsburgh Sleep Quality Index (PSQI), Chen et al demonstrated that constipation was independently associated with poor overall sleep quality (PSQI > 5) in a sample of middle-aged patients. Reference Chen, Bair and Chang17 Cremonini et al, using questions from the Insomnia Severity Index, demonstrated that waking up more than once a night was more likely in the presence of constipation in middle-aged adults. Reference Cremonini, Camilleri, Zinsmeister, Herrick, Beebe and Talley18 Bouchoucha et al. demonstrated that middle-aged patients with self-reported severe insomnia (waking up 1-2 hours early and unable to return to sleep) had a significantly greater prevalence of constipation. Reference Bouchoucha, Mary, Bon, Bejou, Airinei and Benamouzig13 A similar result was obtained by Ueki et al. using the Athens Insomnia Scale, as elderly patients with constipation had significantly higher scores than healthy patients. Reference Ueki, Nagai and Mizukami14 Furthermore, since SE, WASO, and TST were affected by laxative use but not SOL or AI, this may imply that constipation or laxative use is associated with sleep maintenance rather than sleep-onset insomnia.
Laxatives are a widely used treatment for chronic constipation Reference Werth and Christopher6,Reference Johanson and Kralstein7 particularly when diet changes such as increased fiber intake or bulk-forming agents are insufficient. Reference Ramkumar and Rao34 Laxative classes have different mechanisms of action and varying degrees of effectiveness. Stool softeners, for example, are considered emollient laxatives which reduce the surface tension of the stool attracting water to soften them. Reference Wilson and Dickinson35 Severe side effects with stool softener use are uncommon. Reference Wilson and Dickinson35 Osmotic laxatives draw water into the bowels to help treat constipation symptoms Reference Luthra, Camilleri, Burr, Quigley, Black and Ford36,Reference Nelson, Camilleri and Chirapongsathorn37 ; however, prescribed medications of this class such as Linaclotide can have more severe side effects. The United States Food and Drug Administration (FDA) issued a black box label for the prescription of Linaclotide due to the risk of severe dehydration. Reference Rothstein and Friedenberg38 Lastly, stimulant laxatives treat more severe constipation by stimulating the colonic musculature directly. Reference Luthra, Camilleri, Burr, Quigley, Black and Ford36,Reference Nelson, Camilleri and Chirapongsathorn37 Preceding defecation, high amplitude propagated contractions (HAPC) transfer colonic contents over long distances. Reference Duboc, Coffin and Siproudhis8 Patients with constipation have fewer spontaneous HAPCs than healthy patients Reference Ancha, Fajardo and Bauman39 and stimulant laxatives, which act directly on the intestinal musculature, can elicit HAPCs which are quantitatively similar to naturally occurring HAPCs in healthy patients. Reference Bassotti, Gaburri and Imbimbo40–Reference Min, Ko and Kim42 Similar to stool softeners, severe side effects of stimulant laxatives are uncommon. Reference Luthra, Camilleri, Burr, Quigley, Black and Ford36,Reference Nelson, Camilleri and Chirapongsathorn37
It appeared that osmotic laxative users had relatively unaffected markers of sleep continuity, unlike the other laxative user groups. It is possible that the use of osmotic laxatives could ameliorate the effects of constipation on sleep continuity in contrast to the other laxative types. However, the observed difference in objective sleep continuity between different laxative user groups may also be due to differences in sample size available as demonstrated by the large confidence intervals of effect estimates (Table 3). It may have been that more patients were using stimulant laxatives such as Senokot and Bisacodyl since they are easier to obtain (over the counter), whereas common osmotic laxatives such as linaclotide and plecanatide are obtained through prescription. 43,44 Conversely, since laxatives may not be included on a patient’s medication list unless prescribed, it may be that some laxative types which are more likely to be prescribed are overrepresented in our analyses than laxative types which are bought over the counter and may not be reported. Thus, laxative use may be subject to recall bias, as it was recorded based on patient self-report. Regardless, in this middle-aged/elderly population it appears that laxative use was either unable to ameliorate the impacts of constipation on sleep metrics or that use of these agents contributed to poor sleep outcomes.
This study has several limitations. First, objective assessments of colonic transit and anorectal tests were not available in classifying constipated patients or evaluating constipation severity; the presence of constipation was inferred by self-reported laxative use. Thus, we were unable to control for unreported constipation (not using laxatives) which may bias our analysis. Moreover, frequency of usage/dosage of laxative medications was not available. The presence of insomnia symptoms was also based on simple self-report which may not distinguish other subjective sleep complaints. In addition, unmeasured confounders such as socioeconomic status, depression, and anxiety which affect both sleep and gastrointestinal function may impact our findings. Generalizability of results may be limited due to all patient polysomnography reports used in this study being collected from a single site. Finally, the cross-sectional nature of our study prevents us from establishing causal inferences based on these results. The strengths of this study include the ability to objectively assess several sleep parameters using polysomnography in a large sample of patients.
In summary, to our knowledge, this is the first study to investigate the association between various laxative types with objective (polysomnography-derived) sleep parameters. We demonstrate that laxative use was associated with worse sleep continuity, and this was also observed in the stool softener and stimulant laxative subtypes in middle-aged/elderly patients. Additionally, we demonstrate that patients using laxatives (all types) and stimulant laxatives were at greater odds of reporting insomnia symptoms. The results of this study, using laxative use as a surrogate for constipation, support previous findings on the relationship between constipation and poor sleep continuity and an association with insomnia. The findings of this study support the need for physicians to recognize the potential for poor sleep continuity in patients with chronic constipation and facilitate the reduction of poor sleep outcomes through appropriate management. As our study was cross-sectional, we recommend that future studies adopt a prospective study design to investigate how treatment of constipation impacts PSG-derived markers of sleep.
Supplementary Material
To view supplementary material for this article, please visit http://doi.org/10.1017/cjn.2022.264.
Acknowledgments
The authors thank the sleep technologists of the Sunnybrook Health Sciences Centre sleep laboratory for their scoring of the polysomnograms and the support received from the Mahaffy Family Research Fund.
Funding
Dr Boulos’s research program received support from the Mahaffy Family Research Fund.
Disclosures
YSC and BJM have no relationships/activities/interests to disclose. MIB would like to disclose support from the Mahaffy Family Research Fund, Slamen-Fast New Initiatives in Neurology Award from the University of Toronto, Education Research and Scholarship Grant from the Sunnybrook Education Advisory Council and Education Research Unity, Canadian Institutes of Health Research, Innovation Fund from the Alternative Funding Plan from the Academic Health Sciences Centres of Ontario, and the Catalyst Grant from the Canadian Partnership for Stroke Recovery to support MIB’s research program. MIB also discloses support from Jazz Pharmaceuticals for speaker fees and Paladin Labs in honoraria for serving on the scientific advisory committee and speaker fees. MIB also discloses in-kind support (medical equipment) from Braebon Medical Corporation and Interaxon for MIB’s research program.
Statement of authorship
YSC played a major role in the acquisition of data, interpreted the data, and drafted and revised the manuscript for intellectual content. BJM handled design and conceptualization of study and revised the manuscript for intellectual content. MIB handled design and conceptualization of study, interpreted the data, drafted and revised the manuscript for intellectual content, and had supervision of the overall coordination of study.







